On the Way toward Personalized Medicine. Problems of Drug Therapy of Oncological Diseases
In the autumn of 2010 headlines like “Aspirin a day helps to keep cancer at bay, scientists say” hit the world media. It appeared that all of these were inspired by a series of articles in “The Lancet” journal. According to the researchers, a regular taking of small doses of aspirin reduces mortality from certain types of cancer (such as bowel, lung and prostate cancer).
The present paper will consider this phenomenon. The widespread reaction of the public and media to the sensational news has persuaded us to write this paper
Today the function of a physician is reduced to “fitting” the patient into a certain template-diagnosis and prescribing the tablets corresponding to the chosen template
V. Sinelnikov “Love thy Disease of Yours”
Today you can hardly find a person who is not aware of the problem called “cancer”. Judging by the information, found in the mass media, about the achievements in combating this “plague of modern times,“ one can get an impression that the battle with this disease is almost won or, at least, a decisive victory has almost been achieved. Unfortunately, this impression is illusory.
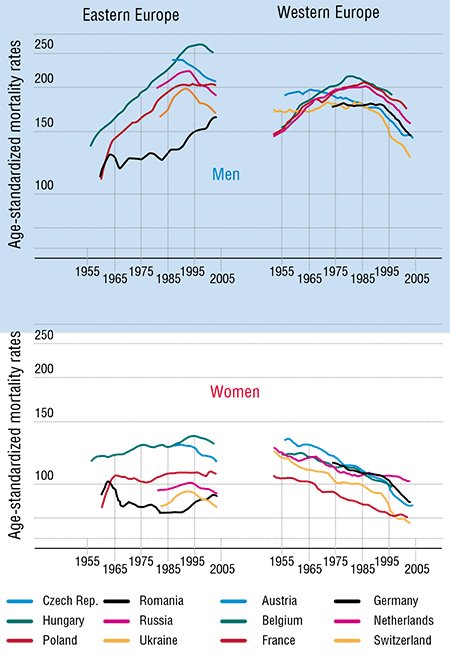
In order to assess the scale of a disaster called cancer, let us take a look at the statistics, obtained, for instance, by the European project FACT (Fighting Against Cancer Today) and published in 2008 (Coleman et al., 2008). Another source is The European Journal of Cancer, whose July 2009 issue is entirely devoted to the results of the EUROCARE-4 Program. These publications present the statistics on cancer incidence rates, cancer patient treatment and survival rates in more than 300 participating organizations.
According to the experts’ opinions, more than 3 million new diagnosed cancer cases were expected in 2009 in the EU countries. This number is steadily growing from year to year. It is estimated that in the EU 2 million people annually die of cancer and its consequences. Moreover, almost a quarter of worldwide cancer cases are registered in Europe with its less than one-eighth of the world population.
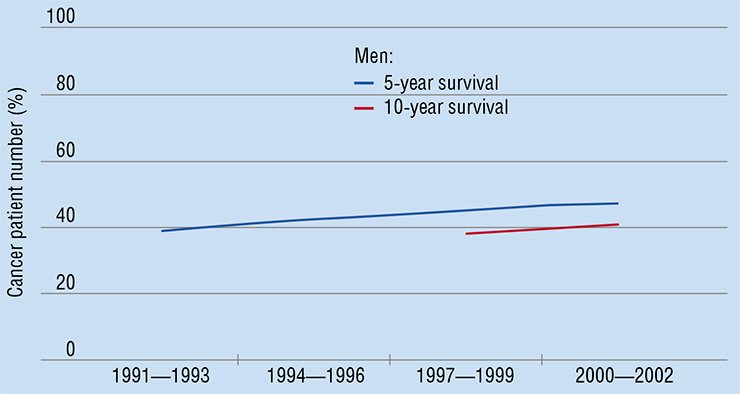
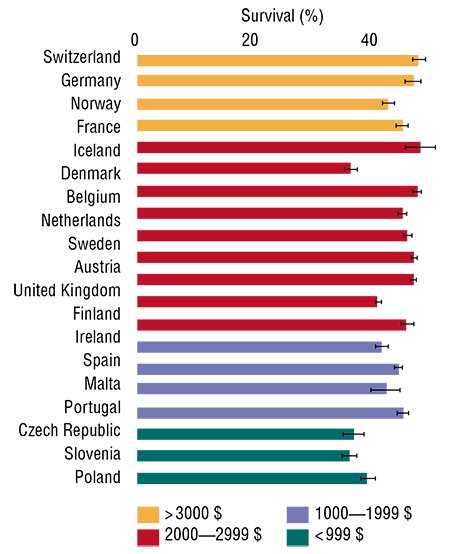
What is the present state of curing cancer? In the last decade, cancer curativeness in Europe has grown by 2 % to 7 % (depending on the cancer type, localization, etc.). By using all modern technologies, it has been possible to extend the life of cancer patients by 6—18 weeks on average. The average survival period of the cancer patient in Europe is now 0.8—2.4 years, depending on the cancer type. These are the figures for the relatively wealthy European countries! Money does not help much in this case though. For example, Switzerland’s expenditure for health care is 10 times higher per capita than in Poland. However, the number of cancer patients who have lived 5 years and longer after they were diagnosed constitutes only 15—20 %.
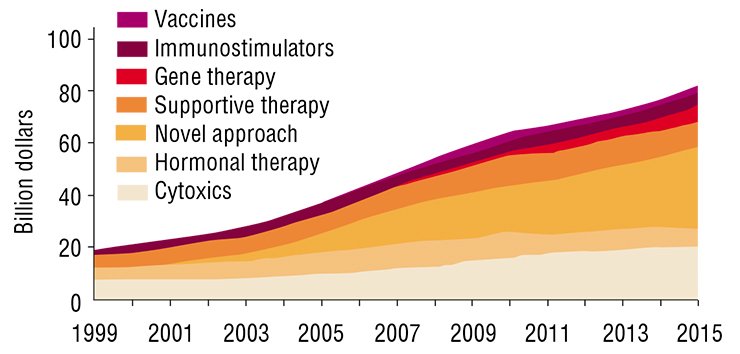
There are certain achievements. But, according to the results of the cited research, the majority of these achievements are due to preventive measures and earlier diagnostics (e.g., wide cancer screening programs) rather than progress in therapies. At the same time, the number of new cancer treatment methods and the number of new cancer drugs grow explosively. In 2007, the worldwide expenditures for cancer drugs were estimated to reach $31 billion (up to 60 % of this amount was spent in the USA). If the existing trend persists, the amount will be ten times greater in 2027.
What is the essence of the problem? Why is the progress in curing cancer patients sharply disproportional to the resources spent? Many specialists have come to the conclusion that the existing approaches to cancer treatment are insufficiently effective, and it is necessary to develop new methods. One experienced practising physician said: “What do you expect? Drug treatment for cancer is still at the stone-age level. We give strong poison to a patient and hope that the disease will die first.”
The devil is in the details
What can be suggested instead of the existing cancer treatment methods? Let us imagine a physician who has to treat a cancer patient. What treatment methods can be applied? Firstly, there is surgery, which is still the most effective way to treat cancer. Secondly, there are therapeutic methods, the ones we shall discuss. There are only three main “tools”: chemotherapy, radiation therapy (treatment using various beams of ionizing radiation), and radionuclide therapy (when radioactive substances are injected into the blood circulation).
Modern chemotherapy is dominated by the so-called cytotoxic drugs, which kill the cells. But there is also a significant number of chemotherapeutic drugs with other types of action. As for radiation-related therapy, the one used most frequently is radiation-beam treatment. Besides, radionuclide treatment is on the increase. There appear other methods, too. For instance, photodynamic therapy (PDT) is continuously gaining strength. In this method a neutral chemical is injected into the blood or the tissue and activated for the therapeutic action by a laser beam. However, as for its action type, PDT treatment is a version of chemotherapy.
The above mentioned therapeutic “tools” have been developed for an average patient, but the physician must prescribe a treatment for a particular person. What can help the doctor to choose a specific treatment for a specific patient? All the data within the clinical trials for the new drugs are processed statistically, in accordance with the existing rules. Researchers try the new drug on patients who were given a similar diagnosis. The approved drug comes to practising doctors with the approved “administration scheme” (regimen) accompanied with a summary on possible adverse effects. So, if the drug has proved to be effective in similar cases, we just use it. What is the problem then? They say that the devil is always in the details. And the seeming advantages of the traditional statistical approach are questionable, say the least.
 According to the fundamentals of statistics, all the patients involved in clinical trials present, strictly speaking, a statistical sample of some hypothetical patient who is, in terms of the probability theory, a random variable. Statistical samples serve for the experiment-based studies of random variables. Thus, the patients in clinical trials serve for the experiment-based studies of the hypothetical patient who plays the role of a targeted random variable.
According to the fundamentals of statistics, all the patients involved in clinical trials present, strictly speaking, a statistical sample of some hypothetical patient who is, in terms of the probability theory, a random variable. Statistical samples serve for the experiment-based studies of random variables. Thus, the patients in clinical trials serve for the experiment-based studies of the hypothetical patient who plays the role of a targeted random variable.
Each of the clinical-trial patients is of a specific sample value, or, in plain English, is a specific “incarnation” of the hypothetical patient. However, the hypothetical patient remains elusive: the results (inherently statistical!) of the clinical trials of a new drug are formulated with respect to the patient who is averaged over those involved in the trials. This results in the drug regimen and related adverse-effect summary. In other words, the fact that the drug is approved means that the regimen and summary are approved.
The approved regimen is supposed to cure/improve the state of the average patient, no matter if such patient exists. The approved regimen even need not cure every patient in the clinical trials. It merely should cure/improve a statistically satisfactory fraction of the patients. To what extent this regimen will be effective for countless future patients is out of the scope of the clinical trials.
The statistical approach to processing the results of clinical trials of drugs leads, in particular, to a profound difference between drug regimens in pharmacotherapy and surgery. A surgeon/dentist “repairs” a specific tissue of a specific patient, whereas a drug regimen is designed to treat the aforementioned average patient who is believed to represent the patients who participated in the clinical trials that were conducted, probably, some decades before.
Statistical methods, in principle, do not allow for the question “why?” An extremely long and expensive developing of a new drug ends in the clinical trials which are based on the trial-and-error method, still the most widespread in medicine. Applying a drug under the same regimen to clinical-trial patients, recording the results, and processing them statistically are still the ways of testing. There is no room to allow for the biochemical characteristics of specific manifestations of the target disease in individual patients. This is probably the reason why the question “why?” is usually omitted. The absence of the answers to the “why”-questions prevents researchers from finding a way to improving the efficacy of the already existing drugs. It also pushes us into developing new drugs, which is again undertaken within the same traditional paradigm of statistical averaging.
One for all, or the individual for each
Once upon a time, in one fairyland, one fairy company (no relation to the real ones whatsoever) decided to manufacture shoes for the local female population. Specifically, they decided to produce “universal” shoes that would fit if not all but, at least, the majority of the population. Clearly, universal shoes would be easier and cheaper to manufacture. After hearing one of the local radio programmes, the management of the company got quite excited: the task could be easier than they had expected! It turned out that the average female foot size in that fairyland was 38. So, going for size 39, or, maybe, 40 (to be on the safe side) would guarantee commercial success.
A trial amount of “universal” shoes was manufactured for due testing. A group of female test subjects of different ages was selected according to the prescribed fairly statistical methods. Initially, it was planned to have 200 subjects (for more reliable statistics), but then the company settled on 100 to keep the trial expenses lower. In the end, as many as 57 females were able to put the shoes on; statistically – a very good result. Some shoes were a little tight, and some were too big. But, based upon the acquired data, the trial was declared extremely successful. As for the unlucky 43 women (their shoes were too small or too large), hopefully, some other company will produce something else.
 The above approach to shoe manufacturing seems rather absurd. However, essentially a similar approach to new cancer drug development does not surprise anyone. Moreover, again and again attempts are made to design the “one for all” blockbuster drug. And every time we are confused when we see that the new drug is not a panacea.
The above approach to shoe manufacturing seems rather absurd. However, essentially a similar approach to new cancer drug development does not surprise anyone. Moreover, again and again attempts are made to design the “one for all” blockbuster drug. And every time we are confused when we see that the new drug is not a panacea.
Another fairyland company, keeping in mind the experience of the first one, concluded that, using the above approach, too large a fraction of the female population was left without shoes. Subsequently, a new “the unique for each” approach was suggested, where each shoe (both the left and the right one) had to fit. It is, undoubtedly, a much better idea, but also a lengthy and expensive undertaking. It is so expensive that not many would be able to afford it, so providing everybody with individually fitting shoes seems impossible in the nearest or, probably, distant future.
In the context of our discussion, such an approach corresponds to the genetic individualization of cancer drugs, when the drugs are not only selected but developed on the basic of thorough knowledge of the individual “genetic makeup” of a particular patient. So far this strategy has not been able to satisfy the drug therapy needs even of a small number of people. According to Prof. Eric C. Lander, the world leader of the International Human Genome Project, an ideal version of genetic drug individualization of which the project participants have dreamed since the start of the project 15 years ago, has worked well only in research presentations and grant applications, so far.
Still another fairyland company decided to use the approach based on preliminary separation of the female population into “uniform” groups, and to manufacture the shoes of a few sizes only (say, 35, 38 and 44). In order to avoid selling a pair of shoes that would not fit the customer, it was decided to manufacture corresponding “express-tests”: namely, insole templates. If the foot fits the template, the customer gets a new pair of shoes. Otherwise, the customer is advised to go to another shop.
Such a “shoe fitting” strategy does not seem less absurd than the first one, discussed above. The so-called “stratification” approach to cancer drug therapy, which is in fact analogous to the insole-template selection, is regarded today as one of the most promising. However, if we extend the idea of “stratification” to its limits, we’ll get the “Cinderella shoe”, i.e. a unique shoe for the only one girl. Modern medicine is not a fairyland prince. It is forced to work with limited resources and, which is even more important, with a distinct lack of time.
Thus, the above three strategies cannot guarantee optimal drug therapy. Is there any reasonable alternative around? Let us again turn to the “shoe” analogy. Suppose you are in a shoe shop with a very good choice. You need to select a pair of shoes that would fit you, but you don’t know your size and you can’t try the shoes on. What would you do? Most probably you’ll ask the shop assistant for advice. An experienced assistant, after estimating your age and build, and after taking the measurements of your foot, will be able to say whether these shoes would be comfortable or not.
The described situation is almost identical to the one in which modern physicians find themselves now. Basing on the available tests and personal experience (both his/her own and the colleagues’), the physician should select for each particular patient the most effective drug regimen from an enormous variety of options. Besides, a reasonable forecast must be made on how the selected regimen will act in this particular case, and with no chance of a “trial run” at that. Drug regimens are not shoes: if they don’t “fit”, it will result in a steady progress of the disease. Moreover, subsequent treatment attempts may be futile because of the time wasted.
Not “what” but “how”
Today many specialists have come to the conclusion that the statistical approach to cancer-drug regimen development and therapy should be updated and become individualized. In this respect, there are only three possible ways to go, as we have partly discussed above. Namely, individualization of the drugs, i. e. development of drugs for each individual case, individualization of the drug regimens, i.e. the schemes of applying the drugs; or the combination of both.
Today it is not clear yet how industrial development of individualized cancer drugs can be achieved. So far nobody has attempted to use the second way. Therefore, the “stratification” approach is assumed to be the most appropriate one (Jørgensen, 2008). Within this approach, patients are supposed to undergo tests which are believed to help to determine if the chosen drug regimen would be effective for them. The corresponding express-test systems existing today are not too expensive and rather easy to use. But, unfortunately, they are not always quantitatively specific and adequate. Besides, they are available only for a minority of drugs and for limited cancer types.
Thus, it would be quite reasonable to explore the second way, the way of individualizing the drug regimens for the existing and widely used cancer drugs. Indeed, there is significant room for manoeuvre even when the approved drug regimens are applied. It could be possible to reach better results at lower expenses, while also reducing an enormous load on, and cost of, the continuous development of new drugs.
We should note that the expenses related to cancer treatment start to dominate when decisions on treatment choice are made. The healthcare systems in many European countries start to limit the growth of such expenses. In many cases, state-owned healthcare systems agree to cover the expenses only for “effective treatments”: the so-called “pay-for-performance” is on the rise. Such schemes have already started to work in the National Healthcare System (UK); in Switzerland only the treatments with a proven effect will be paid for, according to the state law.
Unfortunately, the cancer treatment situation in the EU countries has become worse of late, partly as a result of introducing stricter procedures for the drug/regimen approval. Clinical trials have become more expensive. This, in turn, has caused a decrease in commercial and academic medical research. The latter has led to the growing prices of new cancer drugs. Concerning these aspects, the 2010 Nobel Prize winner in physics A. Geim in his inaugural lecture in Stockholm said: “ ... how many experiments that have led to Nobel Prizes would have been stopped if the ethical, medical and safety regulations at the time had been as strict as they are today? I think too many...”
It is time to return to the “aspirin riddle,” according to which small doses of aspirin “can lower the risk of dying of cancer.” Why did this statement arise such an active interest ? The positive effect of anti-inflammatory drugs on tumours and inflammatory infiltrates has been known for years. Probably, poor availability of inexpensive but effective cancer drugs and slow progress in cancer treatment are negatively affecting the quality of life.
Many people would like to believe in miracles. So it is high time we revised the conventional, intuitive attitude towards expensive new drugs, which are automatically regarded as more trustworthy. Definitely, it is reasonable to spare no effort in increasing the efficacy of the already available drugs.
Problems of an average patient
Let us come back to the average patient strategy and consider it, taking into account body temperature. As is well known, body temperature serves as a common indicator of human health. We know that the body temperature of an average healthy adult is 36.6 °C. However, even a-few-degree deviation of the body temperature from this value cannot tell the physician what exactly is wrong with the patient, and what treatment should be applied.
As noted above, in the field of drug therapy of tumours and inflammatory infiltrates, the “average” patient paradigm should be changed to the individual patient paradigm, the one where specificity and individuality of the disease and of the patient hosting it is taken into account. There are many arguments why it should be so. For instance, it has been proved that imbalance in a diet can lead to cancer. Permanent stresses, little exercise, and even seasonal changes can play a significant role in carcinogenesis (Basso et al., 1992). On the other hand, there is a lot of examples when progress in cancer treatment was achieved through herbal remedies, hypnosis and other types of complementary or alternative therapy. These cases cannot always be subjected to conventional medicine reasoning.
Unfortunately, oncologists have no other choice than to use what is available, i.e. drug regimens designed with a statistically averaged patient in mind. Using a loose translation of a well-known Russian expression, we can say that they have to rely upon the average temperature for the whole hospital.
Generally speaking, the statistical approach to drug development could be regarded acceptable, though with certain modifications. As it is now, it has very significant drawbacks. Some of them were discussed in the above sections. Another group of drawbacks is associated with inherent dependence of the vital parameters on time: the patient today is not the same as he/she was yesterday or will be tomorrow, before or after taking drugs. Actually, each parameter with which we deal in the statistical paradigm can be properly regarded only in the context of time-dependent random variables, i.e. the so-called stochastic processes. This will, if properly allowed for, substantially update the common statistical apparatus to the one necessary for stochastic-process interpretation.
We can expect objections, generally arriving at the following.“ Since there are no other approaches, you have to accept the existing one.” We do not agree either to the first or to the second part of this statement. Firstly, as we will show, alternative approaches do exist. And, secondly, if one does not aim at the improvements, anything at all will hardly change for the better. Today’s critical situation in the drug therapies of cancer demands urgent actions. Actually, there is no need to abandon the methods which may sometimes be effective. However, the overall progress in the field can be achieved only by combining all methods that work.
The traditional “statistical” approach also dominates in the pre-clinical and clinical trials of the new drug as well as in the drug approval process. We have already seen that it misses the target in the case of human patients. Does it work better in the pre-clinical phase, when the drugs being developed are tested on the laboratory animals? Unfortunately, it does not.
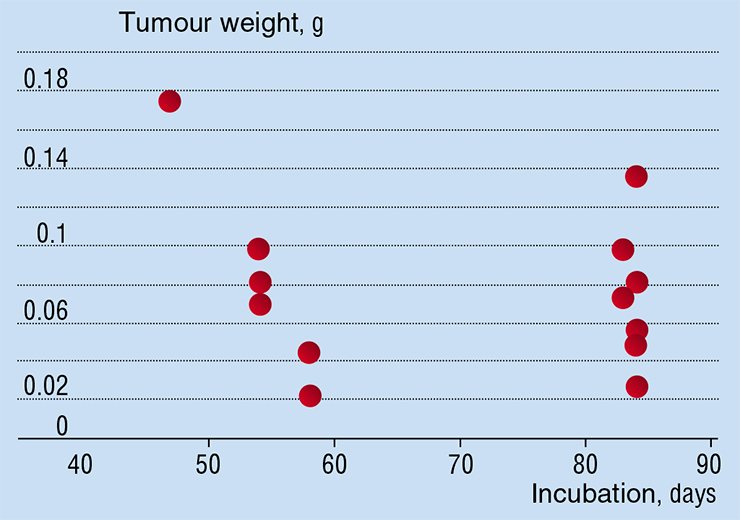
For instance, the general opinion is that laboratory animal lines used for the biochemical and medical research consist of “identical” animals, like clones or, at least, identical twins. Thus, it is assumed that the tumours which are simultaneously “seeded” according to the same standard procedure develop identically. But the results of experiments on the laboratory mice explicitly refute the hypothesis about identity. Indeed, the experiments show that the size of tumours is different for different mice in the same group on the same incubation day. Moreover, it is not guaranteed that the size of tumour on an earlier incubation day will be smaller than the one on a later incubation day. Thus, there is no reason to assume that tumours develop in the same way, even for laboratory animals in the same test group.
If it is not possible to “try the shoes on,” or, in the present context, to forecast how well a drug regimen will work for a specific patient, the way to personalizing the drug therapy seems to be closed. But the well-known many-decade success of mathematical modelling in other disciplines prompts a completely different conclusion: the way in question is actually open. One easily understands the reasons for this success. First of all, modelling is exactly the right tool for making prognoses, both qualitative and, what is more important, quantitative. Secondly, computer technologies allow us to operate large amounts of data that are necessary for modelling complex phenomena such as tumour dynamics. And, thirdly, only modern computers can be suitable means for user-friendly presentation of the results of lengthy calculations.
This is exactly the way in which modern engineers design airplanes. The future airplanes do flights before they are manufactured: virtual flights in the virtual sky. Architects design new buildings and test if they will stand an earthquake without exposing the buildings to an actual disaster, i.e. in the virtual world of computer models. What if physicians get similar tools capable of predicting the drug-regimen action for specific patients with specific diseases? Such systems would allow physicians to make multiple virtual tests of the efficacy of drug regimens in order to “design” the most promising regimen individually for each patient, and, moreover, to do that fast.
In issues to come, we will explain how mathematical modelling can help in individualizing cancer drug regimens. We will also discuss the “tools” which can be designed using this approach, as well as some of the results achieved within almost a decade that we have devoted to developing the approach.
References
Basso A. M., Depiante-Depaoli M., and Molina V. A., Chronic variable stress facilitates tumoral growth: reversal by imipramine administration // Life Sciences. 1992 . V. 50. P. 1789—1796.
Coleman M. P., Alexe D.-M., Albreht T., and Mckee M. Responding to the Challenge of Cancer in Europe // Institute of Public Health of the Republic of Slovenia. Ljubljana. 2008.
J rgensen J. T. Are we approaching the post-blockbuster era? – Pharmacodiagnostics and rational drug development // Expert Review of Molecular Diagnostics. 2008. V. 8. P. 689—695.
Rothwell P. M., Fowkes F. G. R., Belch J. F. F., et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials // The Lancet, Early Online Publication, 2010. 7 December.
The European Journal of Cancer. 2009. V. 45. Iss. 6.