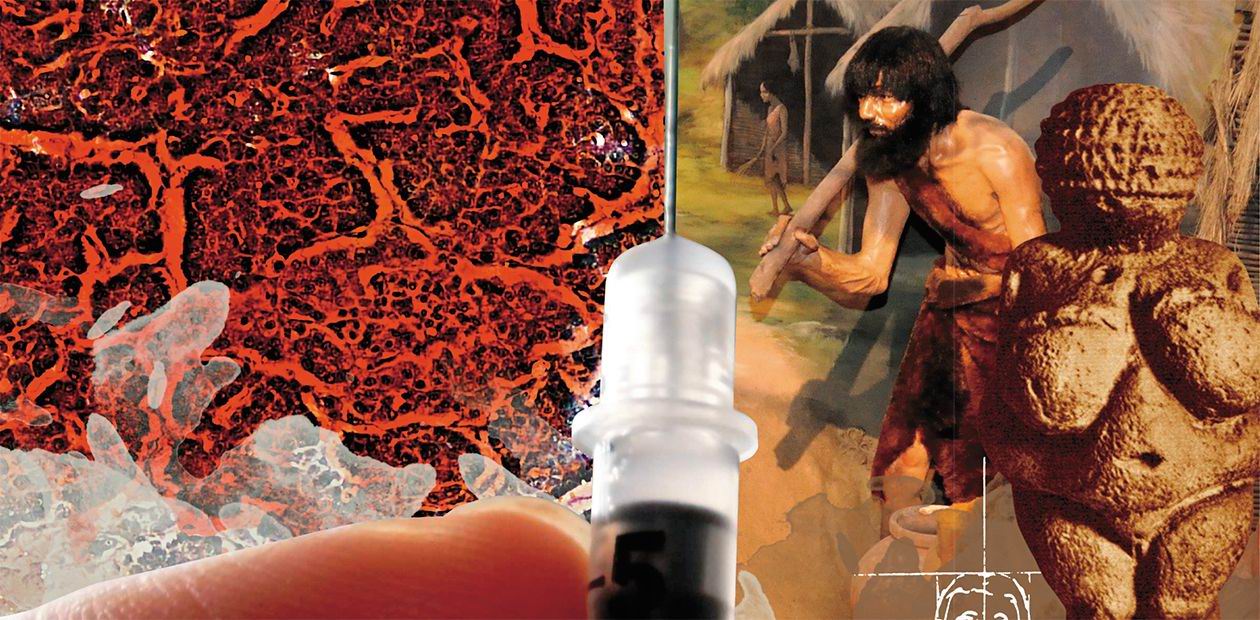
Diabetes – an evolutionary trap?
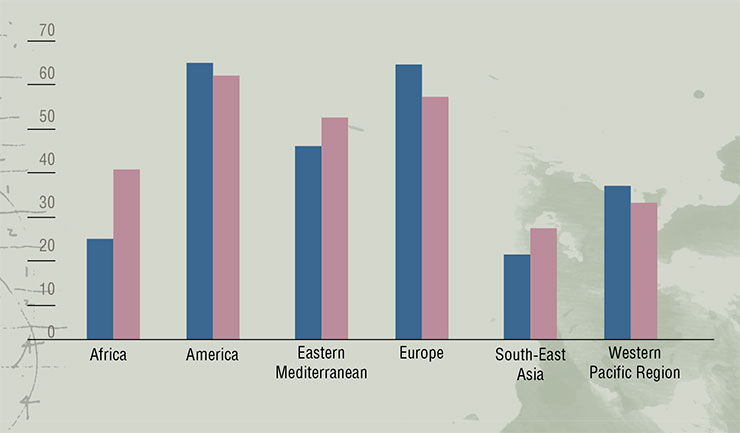
Nowadays, 10% of all healthcare costs go into the fight with diabetes – the number speaks for itself. According to the International Diabetes Federation (IDF), which makes a bi-annual analysis of the current situation and makes a prognosis for the future, every eleventh adult is currently suffering from this disease (a half of all cases goes undiagnosed), and every thirteenth person is at risk of diabetes. Experts predict that by 2025 the number of diabetic patients worldwide will reach 700 million, and every fourth adult in many countries of the world will have to live with this disease
Diabetes… is an affliction not very frequent among men,
when flesh and limbs melt and turn into urine…
If the transition is rapid, the death is speedy.
Life with diabetes is horrible and disgusting…
Areteus of Cappadocia
On the Cure of Chronic Diseases
The number of diabetic patients since 2000, i. e. in the past 20 years, has tripled, and the prognoses that experts publish every other year are becoming more and more menacing. The situation is strange: in contrast to the advances in the treatment of cardiovascular and infectious diseases, diabetes is still spreading at an alarming rate.
How did it happen that diabetes, a non-infectious and once rare disease, is now spreading like an epidemic? To answer this question, let’s have a look at genetic, evolutionary and other factors that could have caused such a conundrum.
Is insulin to blame?
Diabetes, according to the WHO definition, is a group of metabolic diseases characterized by hyperglycemia — increase of blood sugar levels. The reason of this phenomenon lies in the change of secretion and/or efficiency of insulin — a peptide hormone produced by the pancreas. As you can see, insulin is the key factor here.
The very word “diabetes” has been around for over two millennia: it comes from ancient Greek and denotes a condition connected to “passing through”, like water through a siphon. This term is believed to have been coined by Aretaeus of Cappadocia, an outstanding Roman doctor and philosopher, even though in reality he was just the first to describe the condition in detail.He wrote that diabetes was an uncommon ailment where there was “a melting down of the flesh and limbs into urine”. If such melting was fast, it was soon followed by death. From the height of modern knowledge, we must note the remarkably correct description of the main symptoms of the disease, including its essence – the “melting down of the flesh” of limbs, connected to the lack of the all-around anabolic – insulin, and to the hypercatabolic syndrome
There was a long-lasting assumption that insulin is unique to mammals. Eventually, insulin and similar molecules were discovered in almost all vertebrates; by the end of the last century, it was apparent that invertebrates, such as worms and mollusks, also have it. However, in the course of evolution, the functions of these peptides changed drastically.
In more primitive organisms, insulin-like substances generally act as growth factors. Humans also have insulin-like growth factors: IGF‑1 and IGF‑2. However, in the course of evolution, the functions of insulin and insulin-like peptides split and became specialized. The higher the position of a given organism in the evolutionary tree, the more important is the metabolic function of insulin; in humans and higher animals, it has become its primary function.
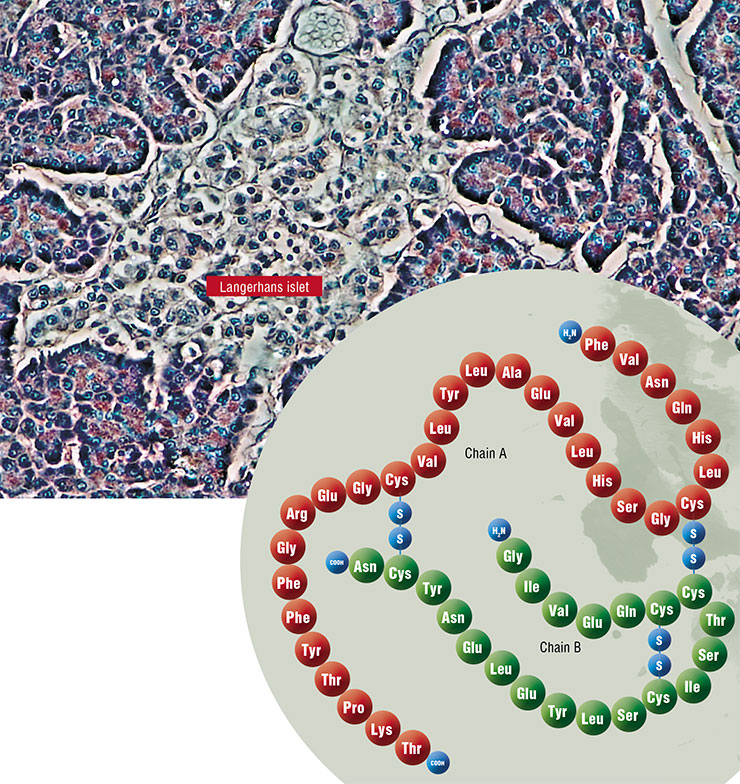
What is this function? Without getting into much detail, we can say that insulin is necessary to deposit excesses of energy substrates, i. e. to facilitate the synthesis of fats, proteins and complex carbohydrates, which are the animal analog of starch in plants — a polysaccharide called glycogen. In other words, insulin is a powerful anabolic agent that enhances metabolic processes aimed at the synthesis of high molecular weight compounds. And vice versa — this hormone suppresses catabolic functions (breakdown of fats and glycogen, and production of glucose from non-carbohydrate compounds). Since insulin is produced after meals, its task is to store whatever has been eaten for the rainy day.
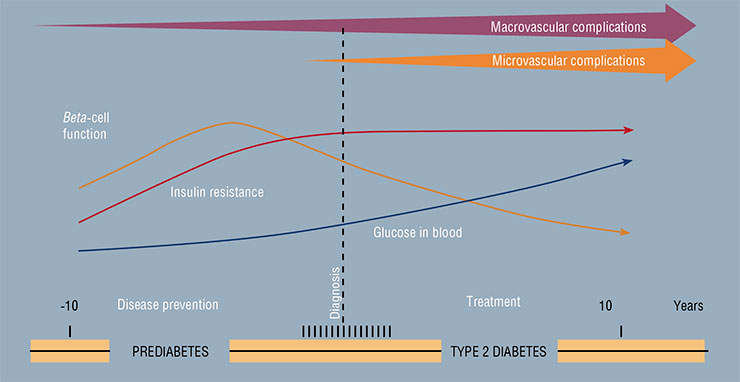
The mechanism of the development of type 2 diabetes, which constitutes the main share of the current epidemic of the disease, is connected with two negative factors: the impairment of cell sensitivity to insulin and dysfunction of beta-cells of the pancreas, which produces this hormone.
The first injection of insulin was performed almost a century ago – in January of 1922. Frederick Banting, a young Canadian scientist, saved a diabetic boy’s life using purified extract of the pancreas. The discovery of insulin in 1921 was acknowledged as one of the greatest discoveries of the 20th century, and Banting received the Nobel Prize together with John Macleod, who was the first to suggest that the reason of diabetes might lie in pancreatic dysfunction and eventually offered his laboratory and his help to Banting. Insulin produced from the pancreas of different animals quickly went into clinical practice – saving millions of lives. In the early 1960’s, insulin was first synthesized chemically, and in 1978, the first genetically engineered human insulin was invented. This recombinant insulin is produced by genetically modified baker’s yeast and E. coliFor a long time, insulin resistance was assumed to be the original cause of diabetes. When the sensitivity to insulin decreases, beta-cells produce larger amounts of this hormone. Eventually, the process fails, and diabetes develops. It is hard to tell whether this assumption is completely correct. It is important to note that the number of people with insulin resistance is greater than the number of those with diabetes. One of the common manifestations of insulin sensitivity reduction is abdominal obesity, when the waist circumference is greater than that of the hips.
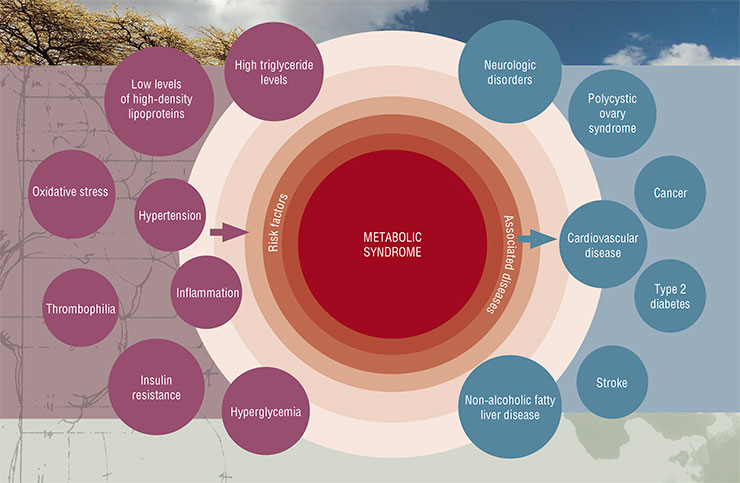
The concept of insulin resistance, better known as the metabolic syndrome, has been extremely popular in medicine for the past 30 years. It covers a large cluster of risk factors, which, apart from the abdominal obesity, include cardiovascular diseases, arterial hypertension, lipid and carbohydrate metabolism disorderes.
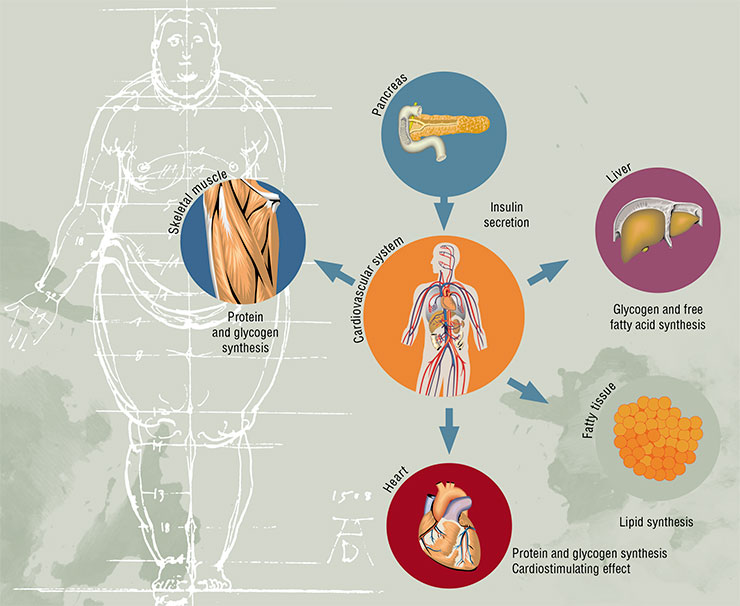
Unfortunately, such elegant and useful concept cannot be viewed as completely confirmed, as there is no clear proof that the insulin resistance, which is at the core of this concept, is indeed its defining element. Unsurprisingly, a number of experts believe that the metabolic syndrome does not even exist. In any case, the decrease of sensitivity to insulin associated with its elevated levels in the bloodstream is undoubtedly closely connected with the main health risk factors of the modern human.
Diabetes genes in evolutionary context
Before 2000, we knew almost nothing about the molecular genetics of diabetes: a few gene polymorphisms (variations of a specific gene) linked to this disease were identified. When wholegenome technologies became available, the results came as a surprise.
First, scientists identified several dozen different genes associated with the onset of diabetes; before that, they were never even considered as possible candidates as their roles were mostly unknown. Second, it turned out that the individual contribution of each of these genes to the diabetic pathogenesis was very little. The latter came as a huge disappointment: it ruined the hope that genetic analysis would become a handy tool for personalized prevention of diabetes. Measuring the patient’s waist and asking about their parents’ health turned out to be a much cheaper and easier tool of predicting diabetes.
This was only the beginning of the story of the molecular bases of diabetes development. Further research demonstrated that “plucking” individual genes out of the whole picture was a waste of time — we had to listen to the whole “orchestra”.
Experts in bioinformatics came to the rescue: they used computers to model the signal pathways underlying the pathogenesis of diabetes. For instance, a group of researchers from the Institute of Cytology and Genetics SB RAS in Novosibirsk studied genetic networks of transcription regulation at the core of diabetes and were able to reveal the key factors involved in the differentiation of beta-cells of the pancreas and adiposetissue (Lashin et al., 2019).
It came as a surprise that the majority of diabetes-related genes turned out to be connected with beta-cell function, and not with sensitivity to insulin. Where were the “insulin resistance genes”, then? If insulin resistance was acquired in the course of evolution, how did it happen, and why did it become so widespread?
By combining the genetic network with the “relative age” of each gene, researchers discovered that they were dealing with an ancient regulatory system, which had been forming along with the development of all life. In other words, type 2 diabetes is based on extremely ancient groups of genes that appeared at very early stages of evolution. In evolutionary terms, diabetes turned out to be close to other pathologies connected with energy metabolism disorders: the Alzheimer’s and Parkinson’s diseases.
The most “ancient” genes turned out to be associated with the monogenic form of diabetes known as maturity-onset diabetes of the young; however, in this case, we are speaking of severe genetic defects. MODY-type diabetes develops in children and adolescents and is clinically similar to premature type 2 diabetes, while usually this disease developsin individuals over 30—40 years old.
Type 1 diabetes, which is an autoimmune disease, is a different story. Its pathogenesis is associated with different, “younger” genes.
The human evolution: adaptive insulin resistance?
The fact that type 2 diabetes is based on genetically defined mechanisms draws our attention to later stages of evolution, and to energy metabolism in the history of hominids in particular.
We know that humans and our closest relative, the chimpanzee, parted their evolutionary ways about 6 million years ago. What were the changes in the lifestyle and nutrition of our ancestors that laid out the foundation for such “civilized diseases” as diabetes?
Let’s begin from the Australopithecus, who led a life typical for higher apes. Its diet consisted primarily of carbohydrates (leaves, herbs, and fruit) and it had to consume large volumes of low-calorie food to get the necessary energy. Its brain volume was a mere 440 cubic centimeters, and none of its artistic masterpieces reached out time — most likely because they were nonexistent in the first place.
The “handy man”, Homo habilis, had a brain volume of about 650 cm3. Its diet was likely to contain more animal proteins and fats, otherwise it would hardly be able to develop a significantly bigger brain, which is a very energy-consuming structure. The diet of the Homo ergaster, which lived 1.8—1.5 milllion years ago, became even more calorie-rich, partially thanks to hunting. As the result, its body became bigger and the brain volume nearly doubled.

Similar tendencies can be seen in the nutrition structure of Homo erectus. With meager paleontological data, we are far from a clear understanding of the selection for genes associated with diabetes throughout this period. Apparently, the requirements to the beta cells of the pancreas began to increase, as high-calorie food required large volumes of insulin, and the role of this hormone in the energy metabolism became progressively more important.
In modern humans (Homo sapiens), the brain has become even bigger. It is estimated to consume up to 40 to 45 percent of all energy generated in the body in the fasting state; generally, it consumes around 20—30 % of the energy produced by the body. This brings up a question: how could evolution follow the path of creating such an energetically imperfect structure? Moreover, it consumes this energy in a very refined form, i. e. as glucose! Apparently, it has to do with adaptation to the changing environment.
There is a hypothesis that insulin resistance is actually the very mechanism that allowed our brain to become more complex and increase in size. Our ancestors’ lives were plagued by hunger and epidemics. We know that in such situations, cells of muscles, liver, fatty tissue and other peripheral organs decrease their sensitivity to insulin — unlike the brain, they can “hold on”. In other words, we are dealing with a mechanism of defense necessary for survival in extreme conditions, which have been established in the course of evolution.
This assumption is indirectly confirmed by the results of research conducted on Jamaica on adults that had gone through marasmus — a major energy deficiency while still in the womb or in early childhood (Francis-Emmanuel et al., 2014). These people turned out to be less sensitive to insulin and had more abnormalities in their carbohydrate metabolism; there were more cases of diabetes e survived because they were initially less sensitive to insulin, which allowed their brain to develop properly.
However, the question of the actual role of insulin resiamong them, too. Usually such changes are explained by disorderes of the development of beta-cells. However, it can be also interpreted from the opposite site: these people may havstance in the development of the hominid brain is still open. Insulin is not just a hormone that improves the way the body uses its glucose; it is also synthesized in certain kinds of neurons and stimulates cognitive function. This agrees well with the evolutionary strategy of shifting from strong to smart, which is assumed to have defined the human dominance over the rest of the animal kingdom, including other hominids.
There is another interesting side to this problem: insulin resistance (at least in men) is also associated with a decrease in testosterone levels, a hormone that is tightly related to aggression. This means that the decrease of insulin resistance may have facilitated the development of more complex social structures in our ancestors.
 More complex social interactions are thought to have played the key role in the evolutionary “victory” of Homo sapiens over Neanderthals. We know that about 30 thousand years ago, Neanderthals virtually disappeared from the Earth, and it happened after yet another wave of migration of Homo sapiens from Africa. At the time of their encounter, the two human species could mix and produce fertile offspring: up to 1—4 % of the modern human genome came from Neanderthals. These Neanderthal fragments, which entered our genome approximately 47—65 thousand years ago, have turned out to be associated with a number of diseases of modern humans, including some autoimmune disorderes, depression, and obesity.
More complex social interactions are thought to have played the key role in the evolutionary “victory” of Homo sapiens over Neanderthals. We know that about 30 thousand years ago, Neanderthals virtually disappeared from the Earth, and it happened after yet another wave of migration of Homo sapiens from Africa. At the time of their encounter, the two human species could mix and produce fertile offspring: up to 1—4 % of the modern human genome came from Neanderthals. These Neanderthal fragments, which entered our genome approximately 47—65 thousand years ago, have turned out to be associated with a number of diseases of modern humans, including some autoimmune disorderes, depression, and obesity.
In 2014, Science published the results of genomic analysis of a large population of native Americans, Mexicans and Latin Americans from Central America, many of whom were diabetic (SIGMA Type 2 Diabetes Consortium, 2014). The researchers were able to isolate a new diabetes-associated genetic locus; “decoding” this locus pointed at its Neanderthal origins. It turned out that this region of the genome is connected with triglyceride levels in the cell cytoplasm — and again, quite likely, with insulin resistance.
Naturally, this discovery does not mean that we “inherited” diabetes from other human species. We cannot assume that the identified locus was “diabetogenic” in the Neanderthal genome: its role could have changed in the course of time. One thing is clear: genetic peculiarities that predispose a person to diabetes have been forming throughout the eons of human evolution.
Neolithic revolution: pros and cons of agriculture
Radical changes in human lifestyle and diet that could have triggered the onset of the diabetes epidemic began about 15—10 thousand years ago. This period, known as the Neolithic agricultural revolution, was marked by the transition of hunters, nomads and gatherers to settled life. The emergence and development of agricultural centers in different parts of the planet led to changes in the human diet structure, such as the steep rise of the share of grains, consumption of milk (unheard of in the Stone Age) and general accessibility of foods.
We are still dealing with some of the consequences of switching from the so-called Stone Age diet to the Neolithic diet. For instance, feeding cow milk to infants became a risk factor for autoimmune diseases, including type 1 diabetes, and intolerance to gluten, a protein in grains, became one of the main forms of food allergy in modern humans.
The most vivid distinction between the diets was the increased consumption of carbohydrates, including fast carbohydrates, accompanied by an equally abrupt decrease in the consumption of fiber. Eventually, products unimaginable to our ancestors came to play important roles in our nutrition, such as refined carbohydrates, vegetable oils, processed grains and dairy products. Nowadays, these products make up to 70 % of our diet, and all of them require rapid and large insulin release from the beta-cells.
What was the likely direction of diabetes-related gene selection in the past few thousand years? Is it possible that our genome has adapted to the changes in our lifestyle and nutrition? Some think that the ten thousand years that have passed since the Neolithic revolution is too short a period for any major adaptive changes in the genome. However, there are known cases of such positive selection — for instance, the genes for lactase, an enzyme that allows grown-ups to digest milk, and amylase, an enzyme necessary for starch metabolism. The Neanderthal genome had a single copy of the amylase gene, while modern humans have numerous copies, which come in handy considering our high-carbohydrate diet.

We should mention the “thrifty genotype” hypothesis proposed in 1962 by James Neel, a renowned American geneticist. This theory explained the accumulation of “diabetic” genes in the human population and increase of the numbers of obese individuals by the fact that our ancestors did not have constant access to food, and natural selection could favor genes that allowed to quickly store up energy. In other words, people who quickly gained weight when food was abundant had better chances of survival during famines.
This theory was popular for several decades, however, it cannot explain some of the facts related to diabetes. First, the relationshipbetween accumulating fat and insulin resistance is not always unambiguous: a number of obese people retain normal insulin sensitivity. Second, to successfully accumulate energy substrates, one needs high sensitivity to insulin and not resistance. This theory also fails to explain why diabetes is extremely rare in native northerners, such as the Sakha and Inuit people. Studies of modern populations with traditional lifestyles (nomads and peasants) show that in the past 10 thousand years, selection was more likely to favor genes “protective” from diabetes (Segurel et al., 2013).
Up until mid-twentieth century, diabetes did not seem to be spreading swiftly despite the apparent changes in the diet structure. It is important to note that most people were still involved in active physical labor: working long hours in the field was hardly easier than going hunting. We know that physical activity is linked to increased insulin sensitivity. Moreover, for the majority of the human population, the problem was lack of food, not its overabundance. These factors postponed the epidemics of obesity and diabetes.
Industrial revolution: fear what you wish
A painting by Peter Bruegel, a famous Dutch artist, depicts a legend popular in medieval Europe. It tells a story of a land where food jumps into people’s mouths without any effort from their side. Back at that time, no one could even imagine this dream to ever come true — moreover, that in some parts of the world, this would happen for the majority of people.
The global-scale catastrophe began after the World War II, when the industrial revolution introduced new changes into the human diet. At last, food became readily, and not sporadically available. Food advertising became widespread. People began to eat more refined carbohydrates, processed grains, products with high saturated fat content, and less fiber; the overall calorie value of food increased. This instantly affected the incidence of obesity, primarily in industrially advanced countries of Europe and the Americas. This is when the diabetes epidemic began.
The effect of consumption of refined carbohydrates on the body contents and on the risk of diabetes is clearly demonstrated by the results of a study on Rhesus macaques that were kept on a diet with excessive fructose content for a year. It caused a drop in the levels of fatty acid oxidation, increase of body weight and fat mass. After six months, their insulin levels in the fasting state nearly doubled, glucose tolerance decreased, and 15 % of them developed diabetes within a year (Bremer et al., 2011).
Of course, in different countries and populations these changes went at different paces. Life itself set up an experiment on one of the indigenous peoples of North America — a tribe called Pima Indians in Arizona. Until mid‑19th century, this population was isolated, and its diet was traditional, based on beans, maize, pumpkins and other vegetables — anything Pima could grow on their land. However, Pima were forced off their land to a small territory in the Gila river valley; the following intensive development of agriculture and avulsion of the river by the settlers made it virtually impossible for the Pima to adhere to their traditional agricultural techniques. This set off what became a famine.
Several decades later, the US government supplied Pima with food aid that consisted of flour, sugar, lard and preserves; combined with low levels of physical activity, this caused rapid spread of obesity and diabetes. In the early 20th century, there was just one registered case of diabetes among the Arizona Pima; by the end of the century, one of every two adult Pima had diabetes. The Arizona Pima turned into the population with the highest levels of type 2 diabetes in the world.
One may wonder: perhaps this rapid epidemic was predefined by certain genetic peculiarities of these people? Yet there is another population of the Pima, which lives in a remote area of Mexico; until 1991, they barely had any contact with the outer world. These people still consume a traditional diet with low fat and high fiber content, accompanied by relatively high levels of physical activity. Studies have shown that representatives of the Mexican population, in comparison to the Arizona Pima, are more sensitive to insulin, and cases of diabetes among them are much rarer (Schulz et al., 2015).
A comparison of the Paleolithic and modern Western diet clearly shows that the Stone Age diet contains less fats, but has a better lipid “profile”. It is associated with a lesser demand for insulin and higher insulin sensitivity as well as lower chance of oxidative stress and inflammation. Women who followed this diet during pregnancy gained less weight, and their babies had lower birth weight. Since pregnancy is a condition characterized by insulin resistance, these women demonstrated the key signs of higher sensitivity to insulinThe Post-Industrial Age provided humanity with high-calorie nutrition, but it came with a drastic decrease in physical activity due to the development of transportation, computers, and the Internet. This created perfect conditions for the spread of the diabetes epidemic. Scientists believe that the following decades will see the greatest increase of the numbers of obese and diabetic patients in Africa and South-East Asia.
Is there a way to prevent the negative scenario? One way to do that is to eat healthy. There is an opinion that we need to “come back to our roots”, and a growing number of experts support the so-called Paleolithic diet. It is characterized by relatively low levels of carbohydrates, higher share of proteins and lack of dairy products. Those who adhere to this “hunter-gatherer” diet have been shown to have lower fat content in the body, and their insulin sensitivity is higher compared to those who follow the usual “Western” diet. However, there is no proven influence of the Paleolithic diet on the metabolic parameters of patients with carbohydrate metabolism disorders compared to other modern physiological variants of diets (such as the Mediterranean diet or the diet recommended for diabetic patients) (Jamka et al., 2020). This means that the return to the Stone Age is probably not worth it.
The second important approach is to increase the levels of physical activity. This is confirmed by the results of a study within the DPP (Diabetes Prevention Program), performed in people with high-risk of type 2 diabetes (Florez et al., 2017). A part of the participants engaged in an almost three year-long program of lifestyle modification, with intensive fitness exercises with a personal trainer and a dietician. The risk of diabetes in these people dropped by almost 60 % compared to those who took a pharmacological placebo. Changes in lifestyle allowed to “overcome” the negative effect of genetic predisposition.
One must bear in mind that the human genome formed in conditions that were very different from the modern-day world. Our genetic program has gone into conflict with our changing lifestyle — and it is failing under the pressure of civilization. Such incongruence paves the way for various degenerative disorders, including type 2 diabetes. Even though the human biology is tuned to a completely different lifestyle, whether we fall into this evolutionary trap completely depends on us.
References
Klimontov V. V., Soldatova G. S. Prophylaxis of diabetes. Novosibirsk: NSU Publishing Center, 2014. 47 p. [in Russian].
Chan C. J., Steiner D. F. Insulin Through the Ages: Phylogeny of a Growth Promoting and Metabolic Regulatory Hormone // Amer Zool. 2000. V. 40. P. 213–222.
International Diabetes Federaion. IDF Diabetes Atlas. 9th ed. 2019. Доступ: https://www.diabetesatlas.org/en/
Francis-Emmanuel P. M., Thompson D. S., Barnett A. T. et al. Glucose Metabolism in Adult Survivors of Severe Acute Malnutrition // J. Clin Endocrinol Metab. 2014. V. 99(6). P. 2233–2240.
Jamka M., Kulczyński B., Juruć A. et al. The Effect of the Paleolithic Diet vs. Healthy Diets on Glucose and Insulin Homeostasis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials // J. Clin Med. 2020. V. 9(2). Pii: E296.
Lashin S. A. et al. Evolutionary Analysis and Mathematical Modeling of Gene Networks of Energy Metabolism Disorders // Systems Biology and Biomedicine (SBioMed-2018): Symposium (21–22 Aug. 2018, Novosibirsk, Russia). Novosibirsk: ICG SB RAS, 2018. P. 78.
Savona-Ventura C., Mogensen C. E. The History of Diabetes Mellitus. ELSEVIER MASSON SAS, France, 2009.
Schulz L. O., Chaudhari L. S. High-Risk Populations: The Pimas of Arizona and Mexico // Curr Obes Rep. 2015. V. 4(1). P. 92–98.
Ségurel L., Austerlitz F., Toupance B. et al. Positive Selection of Protective Variants for Type 2 Diabetes from the Neolithic Onward: a Case Study in Central Asia // Eur J. Hum Genet. 2013. V. 21(10): 1146-51.
SIGMA Type 2 Diabetes Consortium. Sequence Variants in SLC16A11 are a Common Risk Factor for Type 2 Diabetes in Mexico // Nature. 2014. V. 506(7486). P. 97–101.
Wade N. Before Before the Dawn: Recovering the Lost History of Our Ancestors / Penguin Group, 2007. 314 p.
Watve M. G., Yajnik C S. Evolutionary Origins of Insulin Resistance: a Behavioral Switch Hypothesis // BMC Evol Biol. 2007. V. 7. P. 61.