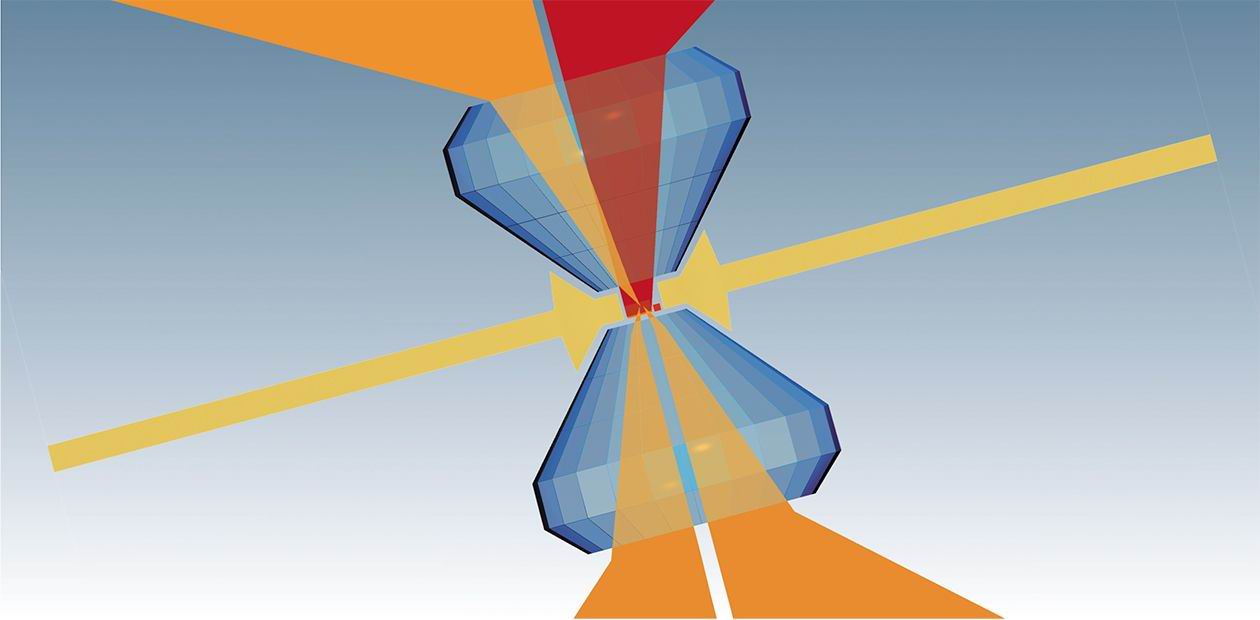
Between the Diamond Anvils
The late 1940s brought about principally new devices allowing for the creation of high pressures under laboratory conditions, namely, diamond anvil cells, booming experimental technology. Such devices weighing only 10—30 g serve to produce pressures up to one million atmospheres; moreover, they require no special physical efforts…
Initially, the area of high pressures was the province of physicists and geologists. Physicists were attracted by the possibility “to test substances” under extreme conditions, where unusual properties can emerge. In particular, under high pressure, nonmetals can be transformed into metals, and electric, magnetic, and optical properties of the matter are changed cardinally.
Geologists were interested in the processes occurring in the matter under conditions close to those actually existing in the interior of the Earth and other planets, the geochemical reactions leading to global geological phenomena, and the processes leading to the formation of minerals. The synthesis of diamonds under high pressures became a particular yet a very challenging problem.
Practical applications of high pressures are manifold: synthesis of new materials, pharmacy (drug production), medicine, and food industry (deactivation of enzymes, toxins, viruses and bacteria)
The late 1940s brought about principally new devices allowing for the creation of high pressures under laboratory conditions, namely, diamond anvil cells, booming experimental technology. Such devices weighing only 10—30 g serve to produce pressures up to one million atmospheres; moreover, they require no special physical efforts. The main principle underlying the operation of such cells is simple: high pressures are obtained due to a very small area whereto the effort is applied.
There are many particular patterns where this principle was implemented; all of them can be conventionally combined into two groups: cells of the piston—cylinder type and of anvil type. As diamond is transparent for infrared radiation, visible and ultraviolet light, x-rays, and neutrons, this makes it possible to study the vibrational spectra and crystalline structures of various substances as well as to monitor their transformations directly under high pressures. Moreover, a specimen may be at a time either heated up to 700 °C or cooled down to a liquid helium temperature.
No wonder that augmenting possibilities drastically increased interest in studying substances under such unusual conditions, which became ever more extreme from experiment to experiment.
In the Arsenal
The first experiments studying the effect of high pressure on chemical and biological objects were performed in the beginning of the last century; studies of minerals started at the same time. For example, the denaturation of chicken protein with increase in pressure was discovered about one century ago.
The first achievements of chemists and biologists on the background of breath-taking successes of physicists and geologists looked rather modest, but it was just a “test of wings”. Really rapid development of high-pressure chemistry and biology started relatively recently.
For chemists, high pressures became a tool for synthesizing new compounds and crystallizing the already known compounds as novel polymorphic modifications, or novel crystalline structures. In addition, high pressures serve to study the nature of chemical bonds and intermolecular interactions, the “flexibility” of molecules, and mechanisms of chemical reactions, including those that are induced not by an increase in pressure, but changes in temperature or irradiation with light.
Biologists apply high pressures to study the dynamics of natural biopolymers — high-molecular compounds forming the basis of all living organisms. Biologists study the factors responsible for the secondary and tertiary structures of these molecules and the corresponding changes occurring during biochemical processes, genetic diseases, and external impacts.
The experiments performed under high pressures make it possible to solve many problems. For example, how a number of organisms (the so-called piezophils) manage to survive in the World ocean’s depths, what processes occur in the living cell during the receptor–substrate interaction, how the muscles work and nerve impulses are transmitted, why the natural fibers — cobweb and silk threads — are so fast, and how to design artificial fibers that are not worse than natural fibers in their durability. Today high pressures are ever wider used also in food industry for deactivating pathogenic microorganisms as an alternative to thermal processing of foodstuffs.
Finally, resistance of organic compounds to high pressures is closely related to the fundamental issue of the natural history — the problem of the origin of life on the Earth, including the possibility of its “importation”.
What are Molecular Crystals?
Crystal is an “ensemble” of atoms displaying the so-called translational symmetry; in other words, this is a set of atoms that, being periodically repeated in space, form a three-dimensional regular structure. All chemical bonds are equivalent in the crystals of cooking salt (NaCl) or diamond (C): it is impossible to find fragments wherein the atoms are stronger bound to one another than to their ambience. The entire crystal of this type is actually one big molecule.
However, crystals with coexisting bonds of different types are much more frequent. Among them are the so-called molecular crystals, where the bonds between individual molecules are weaker than the bonds within the molecules. The molecules in such crystals do not lose their individuality, although the structure and behavior of some of them may change depending on the particular molecular “team”. Despite a relative weakness of intermolecular interactions, it is these interactions that determine the diversity of both the three-dimensional structures that can be built of the same “molecular bricks” and the physicochemical properties of the resulting molecular crystals.
Molecular crystals are applied in electronics and pharmacy and as model objects to simulate the structure and functions of individual fragments of various biopolymers and biochemical systems.
The substances that are liquids or gases under normal conditions can crystallize when the intermolecular interactions are strengthened. This can be reached by either decreasing the temperature (crystallization by freezing) or increasing the pressure (crystallization by compression).
Interestingly, the crystalline structures formed during freezing or compression of the same substance are frequently different. An illustrative example of this is water. The ice formed by freezing has an openwork structure; therefore, its density is lower than the density of liquid water. As for the density of high-pressure ices, numerous examples of which are known, it can be threefold higher than the density of liquid water. With the increase in pressure, the hydrogen atoms become more and more “integrated”: the crystalline structure already does not contain individual water molecules bound to one another by hydrogen bonds but represents an oxygen framework with “common” hydrogen atoms distributed in it.
Different structures are also produced from many other substances — benzene, acetic acid, methanol, ethanol, phenol, sulfuric acid, acetone, and others—depending on the crystallization method. Comparison of these structures serves to better understand the role of various types of intermolecular interactions in crystal formation (including cooperative).
For example, the molecules in crystals of acetic acid form notdimers, as in the gaseous or liquid phases, but endless chains. In this case, the structure yields in the energy of individual hydrogen bonds but gains in the energy of the overall ensemble. The “collective interests” of the team subdue the “needs” of individuals.
High pressures can be used not only for producing crystals of the substances that are liquids under normal conditions, but also for recrystallization of solids. If this substance is first dissolved in a liquid and then compressed, its solubility will decrease with the pressure increase, and crystallization will commence. Thus, it is frequently possible to obtain novel crystalline structures differing from those known at normal pressure.
Some phases of substance that appear due to high pressure can be preserved even after unloading (the so-called “hardening”). This interesting phenomenon is used today in various fields, for example, for the development of new drug forms.
Amino Acid Crystals
Amino acids, small organic molecules, are “bricks” used for the construction of the most important biopolymers — polypeptides and proteins. Characteristic of the structure of these biopolymers are a particular sequence of amino acids (primary structure) and a specific three-dimensional “folding” of the molecules determined by supplementary interactions (for example, hydrogen bonds) between individual fragments of polypeptide chains (secondary structure).
However, amino acids can form specific crystalline structures which represent convenient models for the research at the interface of different sciences — physics, chemistry, and biology — as well as certain fields of technology. Many of such crystals are piezoelectrics or display nonlinear optical properties; they are used as drugs or biologically active supplements.
Finally, amino acid crystals can be used as imitations of individual biopolymer fragments. Almost all of them contain “head-to-tail” molecular chains as building blocks. Although the molecules in these structures are bound by weaker hydrogen bonds rather then by covalent bonds, the amino acid chains are highly resistant to various external impacts and retain their structure long after dissolution of the crystals.
Amino acid chains may be regarded as the simplest models of biopolymers — peptides, where the peptide C—N bonds were formed due to the cleavage of water molecules. Some researchers believe that the propensity of amino acids for producing such structures is directly related to the problem of the origin of life. Polyamino acid chains are the backbone of natural polymers, such as cobweb and silk threads, and artificial polymers (nylon).
Studies conducted under high pressures make it possible to quantify the rigidity and strength of the chains constructed of various amino acid molecules as well as of the layers and three-dimensional lattices formed by these chains. An increase in pressure can cause a reorganization of the three-dimensional structures, the so-called polymorphic transformations. The polymorphic transformations can occur in some structures and cannot occur in others. This “ability” of crystal structures is directly connected with the process of folding, i. e., change in the secondary structure of biopolymers.
The polymorphic transformations in amino acid crystals have analogues among the processes occurring in natural biopolymers. In particular, one of the polymorphic transformations in the crystals of the amino acid glycine caused by increased pressure is accompanied by unwinding of triple helices of the crystal lattice into layers. A similar process is described for collagen, a protein of the connective tissue, providing its integrity. There is also a certain analogy between the polymorphic transformations in amino acid crystals and production of amyloids, rigid stable structures connected with certain severe diseases of infectious, inflammatory, and tumor natures.
The research into polymorphic transitions in amino acid crystals as well as in other molecular crystals is complicated by the fact that the traditional approaches, for example, the construction of phase diagrams, are poorly applicable to their description.
Such processes are usually far from being equilibrium. Certain phases are observable only in specific modes, in particular, not when the pressure is increasing, but “on the way back”, during its decrease. Both the speed of pressure increase and the time of exposure to high pressure can influence the course of polymorphic transformations. For example, a particular phase can be formed in the case of a rapid pressure elevation, and a completely different one in the case of a slow process. The liquids and gases that are present in the system, even in trace quantities, can also influence these transformations. Indeed, these specific features complicate the work, but, on the other hand, make it more fascinating.
The research into molecular crystals under high pressures is yet at its very beginning. This field of science promises many interesting discoveries and practical applications; however, the success requires a close collaboration of engineers, physicists, chemists, biologists, and experts in the fields of synthesis, crystallization, and spectral and diffraction research technologies.
The Siberian Branch of the Russian Academy of Sciences has the necessary background: the first moves in this new and most promising direction have been made under an interdisciplinary integrated project.
References
E. V. Boldyreva. High Pressures and Study of Supramolecular Systems. Novosibirsk: Izd. MDEBT NGU, 2002.
High-Pressure Crystallography / ed. A. Katrusiak and P. F. McMillan. — Dordrechter: Kluwer, 2004.
E. V. Boldyreva, High pressures and supramolecular systems. Izv. Ross. Akad. Nauk, Ser. Khim. Nauk, 2004, vol. 53. no. 7, pp. 1315—1324.
E. V. Boldyreva. Crystal of amino acids as a link between chemistry, biology and materials science // Magic, Models and Mysteries of Molecules / ed. J. Boyens. Springer, 2007.