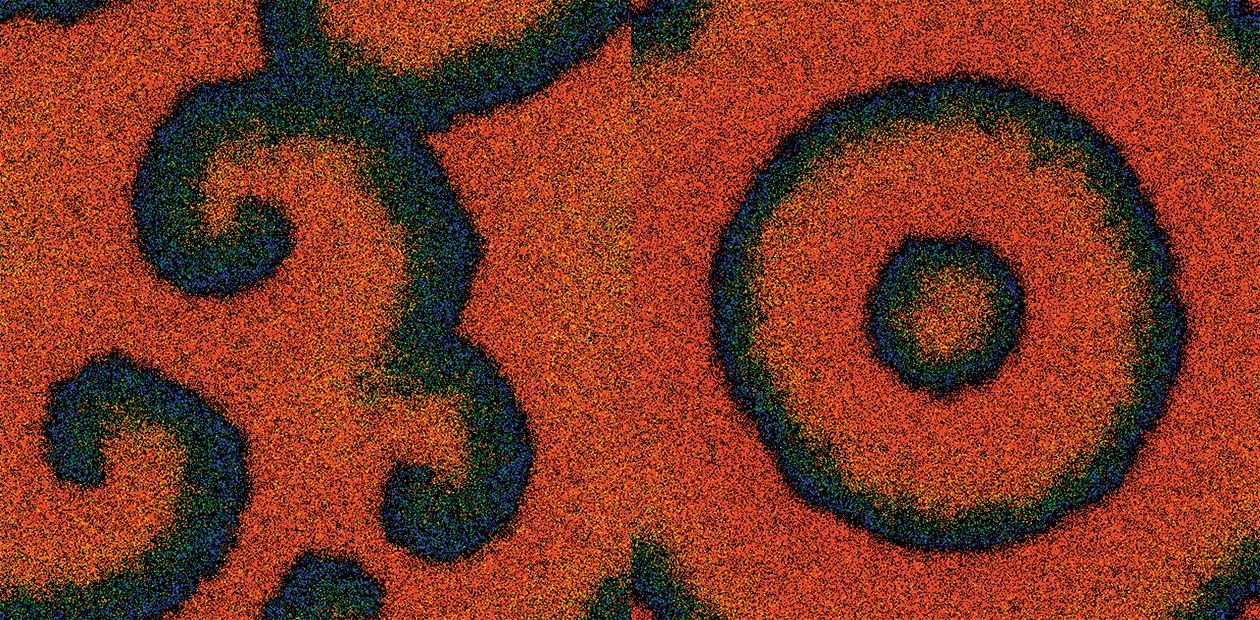
Carbon Monoxide in Color
The G. K. Boreskov prize traditionally awarded to young scientists of the Siberian Branch of the Russian Academy of Sciences (SB RAS) aged under 35 was awarded last year to the researcher of the Boreskov Institute of Catalysis SB RAS, Candidate of Chemistry Andrey Matveev. This distinguished prize was awarded to him for the cycle of studies collectively entitled “Experimental and theoretical investigation of the nature of critical phenomena in catalytic CO oxidation on palladium: hysteresis, oscillations, chemical waves”. The prize winner and his co-authors have managed to show that the unusual phenomena during CO oxidation are caused by the two forms of oxygen: “usual” form adsorbed on the palladium surface and a special “subsurface” form resulting from the diffusion of the oxygen atoms into the topmost layer of the catalyst.
Andrey Matveev became interested in the investigation of physicochemical processes when he was a first-year student at the Physics Department of Novosibirsk State University and attended lectures of the outstanding professor-experimentalist Alexander S. Zolkin. The group was engaged in unusual experiments: obtaining micron-size diamonds by acetylene combustion on a molybdenum support. The experiments were so successful that their results were published in the high-rating journal Carbon.
Andrey Matveev chose the Chair of Chemical Physics for his specialization and after graduation from the University joined the Boreskov Institute of Catalysis, where he began working on the mechanisms of chemical reactions on the surface of catalysts.
It is known that the so-called critical phenomena can appear in catalytic systems under certain conditions. These phenomena include:
– hysteresis, which is a process with an ambiguous dependence of the reaction rate on the external factors, e. g. temperature or pressure;
– oscillations, which are periodic changes of the reaction rate and reagent composition on the catalyst surface in time under constant external parameters – pressure, temperature and gas leakage.
Even more spectacular phenomena are observed in the autooscillation mode under certain conditions. These are periodic in time and space dissipative structures, also called chemical waves.
The investigation of such phenomena allows the scientists to understand deeper and in more detail the mechanisms of chemical reactions. Matveev and his colleagues studied the critical phenomena during catalytic CO oxidation on palladium.
Chemists explain oscillations during CO oxidation on palladium group metals by the formation of two forms of oxygen: active and less active.
The mechanism of oscillations found at relatively high pressures of the reaction mixture (above 1 mm Hg) consists in alternating oxidation and reduction of the catalyst surface. According to the “oxide” mechanism, a drop of the catalyst surface activity is attributable to the blocking of the “sites” for oxygen and carbon monoxide adsorption by the formed oxide. The reduction of the active sites takes place due to slow CO interaction with the inactive oxygen, which is part of the metal oxide. Thus, fast oxidation and slow reduction of the catalyst surface causes transitions between the two steady states of the reaction rate generating the oscillations.
At low pressures (~ 0.01 mm Hg) the oscillations were observed by researchers as early as in the 1980s, in particular, on Pd(110) single crystal. It was assumed that the oxide phase was not formed under these conditions. So, the reaction transition to the oscillation mode was related to the formation of a “subsurface” form of oxygen.
Experiments at the Boreskov Institute of Catalysis SB RAS carried out by X-ray photoelectron spectroscopy have revealed that the oxygen atoms do penetrate into the topmost metal layer forming a special “subsurface” layer. Palladium oxide is not formed in this process. By the way, this fact has a positive effect on the catalytic activity of the surface. Using 180 oxygen isotope and the molecular beam method the researchers have managed to show that the atomic form of oxygen adsorbed on the surface is more reactive than the “subsurface” oxygen form. Periodic formation and consumption of the latter is accompanied by the critical phenomena: hysteresis, oscillations and chemical waves.
A. V. Matveev and his co-authors tend to believe that the “subsurface” oxygen atoms are located in the “valleys” between the rows of the metal atoms on the catalyst surface.
Using a universal Monte–Carlo computer simulation method, they have managed to visualize the adsorption layer and follow the state of the surface in detail. Individual computer frames can be assembled into a short exciting film demonstrating the processes taking place on the surface.
The model of the reaction mechanism includes the following assumptions based on the experimental data: the formation of the “subsurface” oxygen form due to diffusion of the adsorbed oxygen into the subsurface layer and a complex “carbon monoxide on the “subsurface” oxygen”. It appears that there is a range of parameters under which the reaction occurs in the oscillation mode with the formation of chemical waves. Overall, these results are in good agreement with the experiment. The distribution of the reagents during chemical wave propagation becomes exceptionally non-uniform. The change of the adsorption layers occurs in the form of a surface wave with a complex shape. An active reaction between the adsorbed CO and atomic oxygen to form CO2 takes place in its narrow front.
By varying parameters, researchers of the Boreskov Institute of Catalysis have managed to discover a great diversity of spatial structures: rings bands, spirals, “targets”. Many of them were earlier observed in experiments by photoemission electron microscopy.
The obtained results have allowed the researchers both to find out the reasons for the self-organization of the matter at the atomic-molecular scale and to determine a detailed mechanism of CO oxidation on palladium single crystal.
References
Elokhin V. I., Latkin E. I., Matveev A. V., Gorodetskii V. V. // Kinetika i Catalis. – 2009. – V. 50. – P. 40—47.
Elokhin V. I., Latkin E. I., Matveev A. V., Gorodetskii V. V. // Kinetika i Catalis. – 2003. – V. 44. – P. 692—700.
Elokhin V. I., Matveev A. V., Kovalyov E. I., Gorodetskii V. V. // Chem. Eng. J. – 2009.
Ladas S., Imbihl R., Ertl G. // Surf. Sci. – 1989. – V. 219. – P. 88.
Sales В., Turner J., Maple M. // Surf. Sci. – 1982. – V. 114. – P. 381.