Molecules Divided, Loaded, Carried. Nanoporous Polymers in Medicine and Energetics
Siberian chemists have developed an entirely new approach to synthesis of nanoporous metal-organic coordination polymers, which can be widely used in pharmacology, medicine, hydrogen power engineering, and some other spheres. Russia has long been a pioneer in this research field thanks to the efforts of two scientific groups-laboratories at the Institute of Inorganic Chemistry and Institute of Catalysis, Siberian Branch of the Russian Academy of Sciences (SB RAS). Doctor of Chemistry Vladimir Fedin, coordinator of the work on creation of nanoporous structures and Director of the Institute of Inorganic Chemistry, told us about the prospects of the products developed by our chemists
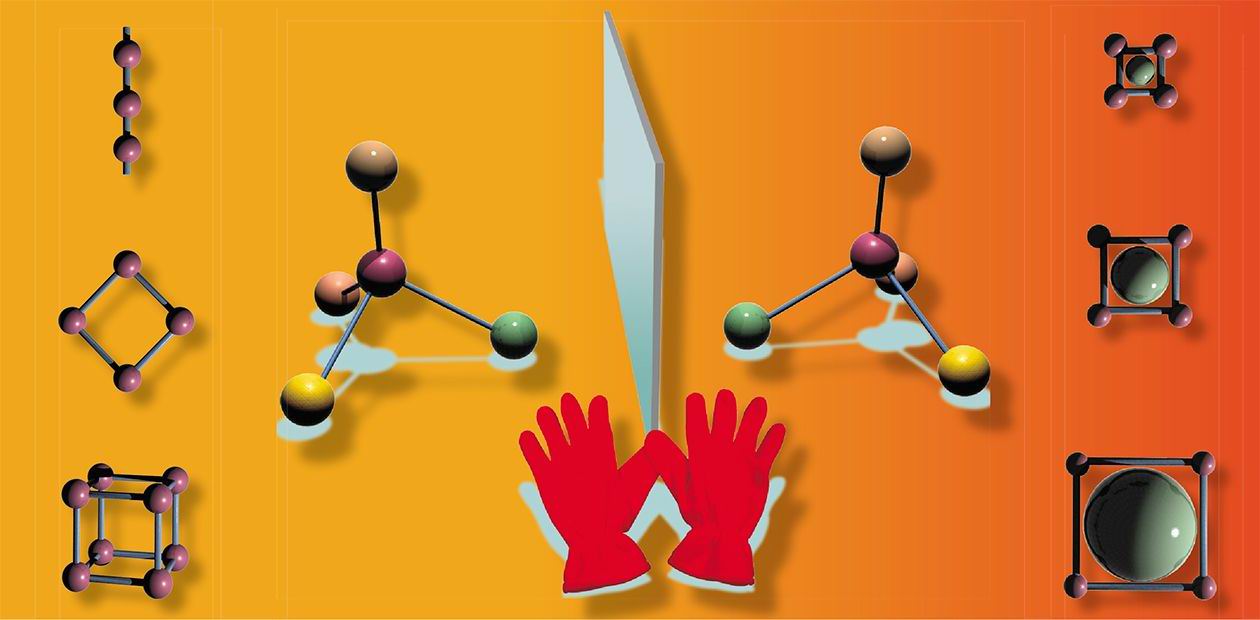
Coordination metal-organic polymers are compounds in which the atoms of metal are alternated with organic ligands (ions or neutral molecules bound to the metallic centers). These structures can be built for many purposes, their chemical design varying from chains to 3-dimensional lattices with the pore size from 1 to 50 nm. Metal-organic structures feature high porosity: up to 70 % of their volume is free space. These polymers are quite effective sorbents: molecules of a certain size and shape are trapped into their pores. They can help, for example, to divide the mixtures of medicines, catching into the metal-organic framework only appropriate molecules.
Dangerous twins
In modern pharmacology, chemical substances sometimes have to be divided into two optical isomers—“left” and “right” molecules. These molecules are perfectly identical in their chemical composition but differ in spatial structure, like a left and a right glove. The property of substances to have two symmetrical isomers is called chirality (from Greek χειρος—hand). These “gloves” behave differently in the human organism. If one molecule of the substance heals it, then the second can damage it irrevocably. This is what happened in the 1960s with thalidomide: its use as a soporific and sedative for pregnant women resulted in thousands of children being born with congenital defects. Another example is ethambutol, used as a basis for anti-tuberculosis preparations. One biologically active isomer of this substance fights tubercle bacilli, and the second provokes blindness.
Molecules of optical isomers are identical in their chemical composition but differ in spatial structure like a left and a right glove. The property of substances to have two symmetrical isomers is called chiralityToday the leading drug manufacturers use only optically pure chiral compounds. But the division of isomers has always been very expensive, so most Russian preparations are still produced as a mixture of isomers (and they are cheaper than their analogues from abroad). However, the “free cheese law” (cheese is only free in a mousetrap) works here, too. It might happen so that one half of a medicine is healing us, while what its twin is doing at the same time in our organism has to be studied separately for each medicine. At best, it does nothing, but we should understand that considering such twins the amount of the active substance is halved.
Chemical “chiromancy”
The metal-organic nanoporous polymers created in the Institute of Inorganic Chemistry are frameworks, traps with a clearly specified structure which cannot catch any types of molecules safe those “hunted”, in the same way as it is impossible to wear a right glove on the left hand. To achieve this, the ligand surrounding of the metallic centers should be chiral.
To divide optical isomers, a mixture of “right” and “left” molecules is run through the chiral coordination polymer. The structure of the polymer should be three-dimensional (for free transport of “guest”-molecules from outside to the inner surface of the pores) and strong (to endure mechanic exposures). In fact, the same polymer framework may be used for synthesis or division of different substances, but in the case when a high-selection division is necessary, the framework must be custom-made for a concrete substance.
When building the coordination polymers in the chemistry institutes of SB RAS, scientists work only with metals and ligands safe for the humans. Fortunately, nature has long solved the problem of division of substances into optical antipodes, so it is senseless to synthesize chirally pure ligands. Malic, ascorbic, citric, camphoric, mandelic, tarataric, and other acids are used as ready-made natural ligands. Copper, zinc, cobalt, and iron are used as metals.
The proposed approach with using the simplest and most available building blocks can decrease the cost of chiral coordination polymers dramatically—they should cost one-tenth to one-hundredth of what we pay for them today. The low cost should become a powerful factor helping a wide introduction of the new materials. The results of the Siberian chemists will find practical use in chemical, pharmaceutical, fragrance, and food industries.
Storage place found for gases
Nanoporous metal-organic polymers can help to solve certain ecological problems. All around the world, CO2 formed at burning oil and coal goes into the atmosphere, but modern American power stations are designed and built so that carbon dioxide can be utilized (kept). In addition to that, in the last five years the world science has been intensely solving the problem of gas preservation for hydrogen power engineering. A number of promising uses of metal-organic coordination polymers is related to their ability to sorb a significant amount of small molecules (for example, hydrogen, methane, acetylene), thus playing the role of vessels for keeping molecules.
Let us imagine that we are ready to produce hydrogen-powered automobiles tomorrow. To carry a cylinder with liquid hydrogen is to carry a bomb. The hydrogen carriers from an alloy of nickel and lanthanum that have been developed lately abroad are good for stationary storing, but are not fit for transportation, since the metals are heavy. To keep 400 kg of hydrogen (this is an approximate amount of fuel consumed in 400 km), an automobile will have to carry a barrel weighing 400 kg! The US Department of Energy and General Motors Company are working hard today on solving the problem of safe keeping of hydrogen. The aim is to reduce gas volume and weight to the minimum and to achieve maximum safety of its transportation. The same problem is to be solved for methane and acetylene.
TARGET DELIVERY OF MOLECULES
An interesting direction of pharmacological research is somehow in tune with the idea of storing and transporting of molecules in hydrogen power engineering. As far back as in 1905, German scientist Robert Berend developed a method for creation of a molecular container—cucurbituril. This structure contains a fairly large intramolecular cavity that can hold a full set of molecules or ions, and its negative ions are able to enter into a reaction and make associates with the positive ones. The top and the bottom of the container’s “barrel” is covered with clusters -“lids”, which can be opened and closed due to forming or breaking of hydrogen bonds. The cucurbituril can help in target delivery of medicines, which are unpacked by cue, reacting to the change of the pH of the medium in which they are found. In particular, it may become possible to cure cancer tumors by delivering to them in the containers platinum complexes and other substancesExperts from the Institute of Inorganic Chemistry SB RAS have conducted unique experiments of synthesis and determination of crystal structures of metal-organic polymers with the molecules of methane and acetylene included in the nanopores. The obtained results help to plan a synthesis of new coordination polymers with the improved characteristics for storing combustible gases and CO2.
Recently, the metal-organic polymers have become the most important object of chemical research in the developed countries (the USA, European Union, Japan), and in China. The project financed by the Ministry of Education, Science, Sport, and Culture of Japan can serve as an example of a large national program. Today, the research institutes of SB RAS have established good relations with the scientists from Germany, France, Korea, and Japan. The international collaboration helps to reach the goal—creation of new functional materials based on metal-organic polymers—faster and more effectively.