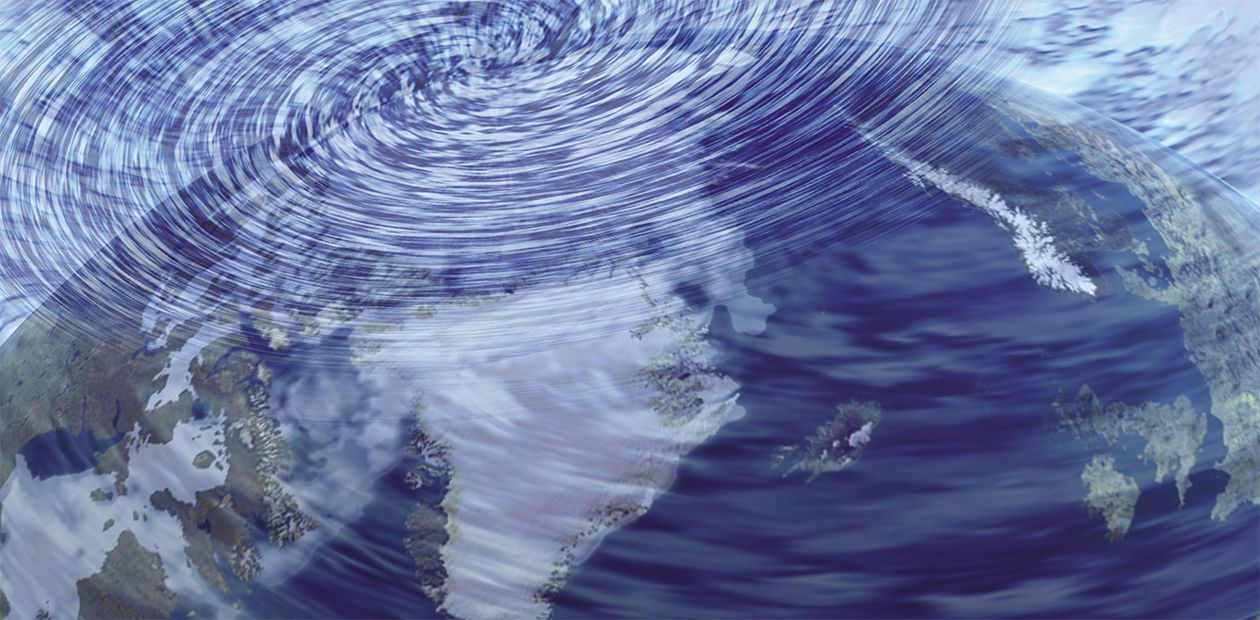
Ozone Holes: Born by Stratospheric Air Flows
But even though it occurs in such small quantities, ozone plays an exceptionally fundamental part in the life on the Earth: It cuts off the short wavelength portion of the solar spectrum, or the ultraviolet (UV) radiation, which destroys proteins and nucleic acids. Furthermore, ozone is an important climate agent responsible for short-term and local weather variations.
Ozone destruction can be sped up by substances that react catalytically. The catalysts can be either natural oxides inherent to the atmosphere or those added as a result of natural hazards like volcanic eruptions. Besides the natural substances, ozone destruction can be catalyzed by industrially manufactured chemicals that reduce the screening capacity of the ozone layer. This fact was discovered in the second half of the 20th century and alarmed both scientists and the broad public...
Ozone is a specific triatomic form of oxygen making about one part in three million of all Earth’s atmospheric gases. But even though it occurs in such small quantities, ozone plays an exceptionally fundamental part in the life on the Earth: It cuts off the short wavelength portion of the solar spectrum, or the ultraviolet (UV) radiation, which destroys proteins and nucleic acids. Therefore, life could exist only in water prior to the advent of photosynthesis and the ensuing appearance of free atmospheric oxygen.
Furthermore, ozone is an important climate agent responsible for short-term and local weather variations. Absorption of solar radiation by ozone and energy transfer to other gases causes quite strong heating of stratosphere and thus controls the planetary-scale thermal and circulation processes in the atmosphere.
The chemically unstable ozone molecules naturally form and break down under the effect of biotic and abiotic agents, and the evolution has brought the process to some balance. Ozone, as well as molecular oxygen, is subject to external effects which cause its decomposition. Ozone destruction can be sped up by substances that react catalytically. The catalysts can be either natural oxides inherent to the atmosphere or those added as a result of natural hazards like volcanic eruptions.
Besides the natural substances, ozone destruction can be catalyzed by industrially manufactured chemicals that reduce the screening capacity of the ozone layer. This fact was discovered in the second half of the 20th century and alarmed both scientists and the broad public. The public opinion on the ozone problem became stirred up especially after the discovery of the so-called ozone hole over Antarctica.
A “hole” over Antarctica
The notable depletion of the ozone layer was first discovered in 1957 during the International Geophysical Year. But the true history of the Antarctic ozone hole began twenty eight years later with the paper by British scientists Joseph Farman, Brian Gardiner, and Jonathan Shanklin published in Nature which reported a drop in total ozone over Antarctica and hypothesized that industrially manufactured gases, including CFCs, were responsible for the spring ozone loss over Antarctica (Farman et al., 1985).
It was found out that the Antarctic ozone hole appears every two years on the average, holds from ninety to one hundred days, and then disappears. It is not an “open hole” as it may seem, but rather a sag of the geometrical surface drawn as DU ozone as a function of geographic coordinates. Unfortunately, further studies of the Antarctic ozone hole mostly aimed at proving its manmade origin (Roan, 1989).
One millimeter of ozone
Atmospheric ozone exists in an about 90 km thick spherical layer immediately above the Earth’s surface. It is distributed unevenly, both vertically and laterally, with the maximum concentrations at a height of 26—27 km in tropic latitudes, 20—21 km in middle latitudes, and 15—17 km in polar regions.Total column ozone (TCO), or total amount of ozone above the station, is measured using the optical properties of ozone that absorbs and backscatters solar radiation in Dobson units. 100 DU is equivalent to a one millimeter thick layer of pure ozone at the sea level temperature and pressure.
Total ozone is subject to diurnal, seasonal, annual, and decadal variations: Natural thickness of the ozone layer varies from 90 to 760 DU, with a global mean of ~290 (DU).
The ozone density is monitored by a world network of about a hundred and fifty land stations which are scattered very unevenly over the continental area and have a very little chance to detect total ozone anomalies even as large as 1000 km. A higher-density monitoring is possible with optical instruments mounted on satellites. A slight ozone layer depletion is not a disaster, especially in the middle and high latitudes. Besides ozone, UV radiation is absorbed by clouds and aerosols. A large part of middle latitudes of the northern hemisphere, for example Central Siberia, where the number of cloudy days is up to 80 % a year, suffers an ultraviolet shortage (about 45 % of the medically required dose).
The numerous existing hypotheses on the formation mechanism of ozone holes attribute its origin either to manmade or natural causes and invoke either chemical or dynamic mechanisms or their joint effect. The theory of manmade chemical origin of the ozone hole is not universally supported as it faces a number of contradictions. For instance, it fails to explain the locally observed increase in stratospheric ozone as the atmospheric CFC contents should build up and destroy the ozone layer. Of special interest is the most naive question: Why should the hole exist in the southern hemisphere when CFCs are produced in the northern one, more so that there are ¬apparently no winds from the northern to southern hemisphere during the hole formation.
The question whether the Antarctic polar stratosphere is indeed isolated when the ozone hole appears — as well as many other related problems — can be solved using the new method suggested by V. B. Kashkin for tracing stratospheric air dynamics (Kashkin and Sukhinin, 2001; Kashkin et al., 2002). The new approach is advantageous over the available ozone-depletion theories of which none is based on global-scale monitoring of total ozone and stratospheric processes.
A notable depletion of the ozone layer was first discovered in 1957, and about thirty years later scientists blamed industrially manufactured gases
The density of stratospheric ozone existing as a large continuous cloud over the earth’s surface varies from place to place and from time to time. This variation pattern is similar to density variations of water clouds on an overcast day. People have used the pattern of an overcast sky as an indicator of tropospheric air flows — translation and rotation — since long ago.
Air flows in the stratosphere, above 10 km, can be traced against the ozone density in the same way as one learns the wind direction from moving clouds. For this total ozone is measured, for example, over the southern hemisphere, on a square grid (from place to place) at two successive times (say, every 24 hours). Comparing the ozone fields of two successive days allows estimating the daily rotation angle, direction, and speed of ozone clouds.
The new method was applied to investigate the ozone layer dynamics and related stratospheric air flows in 2000 when the Antarctic ozone hole was especially large, using satellite total ozone measurements over the whole southern hemisphere from equator to the pole (Kashkin et al., 2002). The measured total ozone was the lowest inside the whirlpool over the pole in the center of a ring-shaped circumpolar vortex, which we discuss below in more detail. That was a prompt for our hypothesis of ozone hole formation by a natural mechanism.
CFC Ban — Who gained?
In 1973, American chemists F. Sherwood Rowland and Mario Molina discovered that Cl atoms released from manmade synthetic chemicals as a result of their breakdown under sunlight can destroy the stratospheric ozone layer. They especially blamed chlorofluorocarbons or CFCs, also called freons, which had broad application in home and car air conditioners, as propellants for dispensing aerosol sprays, blowing or bubbling agents for foam packaging and insulation, cleaning agents, etc. Rowland and Molina published their findings in Nature in 1974, and in 1995 they, together with Paul Crutzen, won the Nobel Prize in chemistry.
The discovery called forth restrictions on the manufacturing and use of ozone-depleting chemicals that became prohibited in many countries worldwide. Today the Montreal Protocol on Substances that Deplete the Ozone Layer, implying control over 95 chemicals, has been signed by more than 180 nations. The Environmental Protection Law of the Russian Federation likewise includes a special article for the ozone layer conservation.
The CFC ban had serious political and economic consequences, as CFCs showed a number of advantages being non-toxic, highly stable, fire-safe, and compatible with many materials. The ban was first met with hostility from chemical industry people, in the US in particular. However, later Du Pont supported the CFC restrictions and suggested hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) as an alternative to CFC, especially, CFC-11 and CFC-12, which cause the greatest damage to the ozone layer.
Then there followed a boom of replacing the old refrigerators and air conditioners by new ones free from ozone-damaging coolants, though the use of HCFC and HFC instead of CFS coolants makes the appliances less efficient and durable, more expensive and power consuming. The companies that were the first to use new coolants got an enormous gain. Losses from the CFC ban amounted to many tens of billion dollars, or more, only in the US. Some people suspected that the ozone-saving policy might have been inspired by the greatest chemical companies like Du Pont and ICI to fight their competitors and to gain more strength on the world market.
Global stratospheric dynamics: a hypothesis
Circumpolar vortices arise during the motion of stratospheric air along meridians and along latitudes traceable by the ozone cloud. That’s how it goes. The stratosphere is higher above the warm equator than above the cold poles and makes a sort of a gradually steepening hill. In its motion from the equator to the pole (south pole in our case), air, together with ozone, rolls downhill accelerating poleward. On the other hand, air flows along latitude (W — E) are driven by the Coriolis force associated with the Earth’s rotation. The resulting air motion looks like spindle-like winding at a small step onto the southern and northern hemispheres as far as the poles.
The winding of stratospheric air occurs all year round but is especially intense every late winter and early spring both in the northern and southern hemispheres. The reason is that the height of the stratosphere above the Earth’s surface is almost invariable at the equator and variable near the pole, being the highest in summer and the lowest in winter when the polar regions are the coldest.
The ozone density in the middle latitudes is produced by quite abundant ozone supply from the equator and in situ photochemical ozone formation. Ozone near the pole is mostly due to supply from the equator and from the middle latitudes. Its density near the pole is low as little ozone forms photochemically under the low-angle sunlight and much of the supplied ozone breaks down during the travel which is longer than ninety days, its half-life.
This is a normal ozone density distribution and air flow pattern on both poles in the absence of ozone holes. However, in the coldest winters the stratosphere goes down very low above the Earth’s surface and the hill is especially steep. Thus, the downhill stratospheric air flow speeds up to produce an effect familiar to everyone who ever saw water flowing out of a tub when the plug is out. Once the outflowing water on the surface reaches some speed, it becomes involved in rapid rotation making a “whirlpool” over the hole due to the vortex-induced centrifugal force.
Something similar occurs with the global motion of stratospheric air flows. As the accelerated stratospheric air rolling downhill gathers enough speed, the centrifugal force pushes it off the pole towards the middle latitudes. The general pattern is that the winding of stratospheric air flows (with ozone) toward the middle latitudes occurs simultaneously in opposite directions: from the equator and from the pole. This facing migration of air toward the middle latitudes produces a rapidly rotating ring with a very high ozone density.
Satellite ozone density data prompted the hypothesis of ozone hole formation by natural mechanisms
The stratospheric air connection between the equator and the pole breaks during the life of the ozone hole. So, the ozone supply from the equator and the middle latitudes breaks as well and, moreover, the existing near-polar ozone becomes pushed off centrifugally, because ozone is heavier than air. As a result, the ozone concentration in the whirlpool inside the vortex ring drops dramatically, which is interpreted as an ozone hole, and the ozone concentration within the ring itself strongly increases.
In late spring, when the stratosphere warms up and rises higher above the surface, the downhill rolling of stratospheric air slows down, the whirlpool disappears, and air connection between the middle and high latitudes becomes bridged again, the poleward ozone supply from the middle latitudes resumes, and the hole heals up till the next extremely cold winter on the south pole.
Although cicumpolar vortices appear in the northern hemisphere as well, large ozone holes never arise at the north pole where winters are never as cold as at the south pole
And what’s in Arctic?
Although the flows of stratospheric air — and, hence, ozone — behave in a generally similar way in the two hemispheres, a large Antarctic ozone hole appears from time to time around the south pole but has no counterpart in the north. That’s primarily because Arctic winters are warmer, and the stratosphere is never as low as to provide the critical speed of the downrolling air required for a whirlpool.
There is another essential point of difference. The W — E air flows in the northern hemisphere are about twice slower than in the southern one. The reason is obvious: Antarctica is surrounded by oceans with a circumpolar oceanic current, where rotation actually involves an enormous mass of water and air together. The northern hemisphere, on the contrary, is mostly continental with large mountain provinces at middle latitudes. Friction of air against the rugged land surface prevents the W — E winds at middle latitudes from gathering enough speed.
Small ozone holes may appear in the northern middle latitudes as well but these are of different origin. The topographic ruggedness in the mountainous continental northern hemisphere produces an air flow pattern at the middle latitudes similar to that of water in rapid shallow rills with a stony bottom where vortex movements form numerous small bosses and whirlpools on the water surface. The bosses and whirlpools appear and disappear on the water surface over the respective bosses and pits on the bottom. For the mid-latitude stratospheric air flows in the northern hemisphere, they are temperature contrasts at the land sea and plainland highland interfaces that play the part of bottom topography.
Rapid stratospheric W — E air flows in response to temperature contrasts on the Earth’s surface cause vertical flows in the troposphere. As a result, stratospheric winds over the vertical flows generate clockwise and counterclockwise vortices. Low-ozone regions (ozone holes) arise inside the vortices, with their characteristic size much smaller than the south pole ozone hole. Note that vortices rotating in different directions were discovered at first search.
Thus, the dynamics of stratospheric air flows traced using the new method against the motion of ozone clouds provides a plausible explanation for the formation of the Antarctic ozone hole. The ozone layer changes are most likely produced by natural processes in the terrestrial atmosphere and apparently existed long before man.
We are far from claiming that CFCs and other industrially manufactured gases cause no damage to the ozone layer. However, it remains unclear which exactly are the shares of natural air-flow and manmade mechanisms responsible for the formation of ozone holes. Anyway, hasty conclusions in such a serios matter are unacceptable.