Death of Prometheus
According to an ancient Greek myth, Prometheus, a Titan, was punished for helping people; for hundreds of years the hero was chained to a rock and an eagle of Zeus tore at his liver every evening. Fortunately, the hero’s liver was restored in the morning thanks to its fabulous regeneration potential known since ancient times. But what will happen if, instead of an eagle’s beak, viruses, alcohol or toxins act on an ordinary person, not on an immortal deity? The answer to this question has been known since the time of Hippocrates: initial apathy is followed by aggression, brain fog, muscle cramp, coma, and eventual death. Currently, this is the fate of one person per several thousand…
Hepatic encephalopathy is a gradual brain dysfunction, a spectrum of neuropsychiatric disturbances (Ferenci et al., 1999, 2002), emerging in response to acute or chronic liver injuries when this organ loses its function of an active filter able to neutralize the toxins incoming from the gastrointestinal tract to the central bloodstream , first and foremost, the ammonium ion (NH4+). Written sources suggest that Hippocrates, who lived two and a half thousand years ago, was the first to notice the correlation between the progress of liver diseases and brain function disturbances. However, such phenomena might have been known to Sumerians, ancient Egyptians, and the Chinese. Indeed, encephalopathy may also be caused by failures of other organs (for example, kidneys), as well as by insufficient blood supply, or a direct brain infection, but these causes are less common.
Several stages of chronic hepatic encephalopathies are distinguished (Ferenci et al., 1999). The zero stage has no clinical or psychometric symptoms. The stage of mild encephalopathy is characterized by a shortened attention span and changes in the brain motor functions detectable only through specifically designed tests. The subsequent stages bring about a range of pronounced psychic, behavioral, and motor disturbances starting from memory problems, confusion, insomnia or somnolence, discoordination, and fatigue through apathy, lethargy, hand tremor, incomprehensible speech, and inappropriate behavior to disorientation in time and space, amnesia, occasional fits of rage, and hyperreflexia, eventually leading to loss of consciousness and reflexes ending in comaModern biomedical research of hepatic encephalopathy dates back to the late 19th century. The subsequent 120 years, full of discoveries and delusions, hard work, mischance of some researchers and good luck of others, partly resembles a century-long story of how Europeans searched for the sea route to India.
From Meat Intoxication Syndrome in Dogs to Human Liver Coma
The very beginning of experimental research into the association between liver diseases and brain dysfunction dates back to 1877, when N. V. Eck, a young Russian military surgeon, designed first vascular anastomosis as the method of the decompression of the portal system through the formation of new ways of the blood outflow from the portal vein into the inferior vena cava (Eck, 1877). However, seven of the eight dogs died during several days after the intervention. Dr. Eck assumed that the negative results of his experiment were predetermined by the imperfect technique of surgical operations on animals.
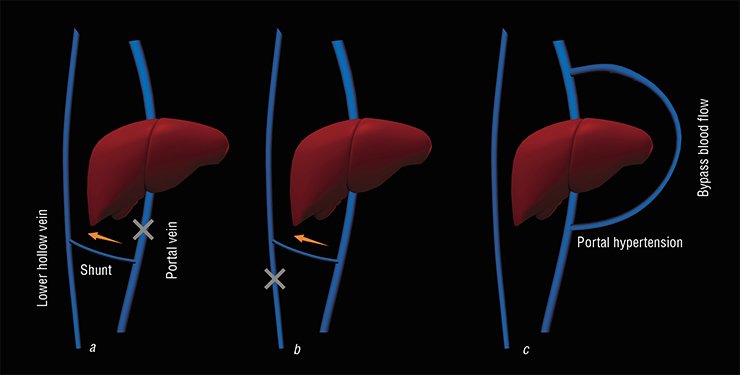
Six years later, I. P. Pavlov, a future Nobel Prize winner, evaluated the impact of Eck’s fistula for further studies of liver physiology, pathophysiology and pharmacology after he had read the article by Dr. Stolnikov published in the Pflugers Archive in 1883. For several years, Ivan Pavlov practiced and improved shunts overlay techniques to obtain reproducible experimental results. Subsequently this method of bypass surgery, well known as Eck’s fistula (or Eck-Pavlov shunt in Russia), was widely applied in the practice of experimental studies of liver functions and hepatic encephalopathy.
The work performed by Pavlov’s group in 1893 (Hahn et al., 1893) is considered as the beginning of systematic research in this field, wherein strange syndrome of “meat intoxication” was demonstrated in the experiments on dogs with a portocaval shunt. The dogs that underwent the surgery appeared not to stand any feed with high protein content. Irritability, aggressiveness, spastic and staggering gait, wandering eye, and blindness rapidly changed to stupor, cramp, coma, and death. However, the symptoms disappeared in the absence of meat (and, correspondingly, nitrogen excess) in the diet. At that time Pavlov worked at the newly founded Imperial Institute for Experimental Medicine in St. Petersburg (A. Nobel had allotted a grant of 10 000 rubles for its equipment, first and foremost for Pavlov’s laboratory; it was a great sum at that time).
In 1895, Pavlov’s collaborator, great polish biochemist and chief chemist of the institute, Marcel Nenski described an improved method of ammonia determination and measured ammonia in the tissues and liquids of experimental animals (Nenski and Zaleski, 1895).

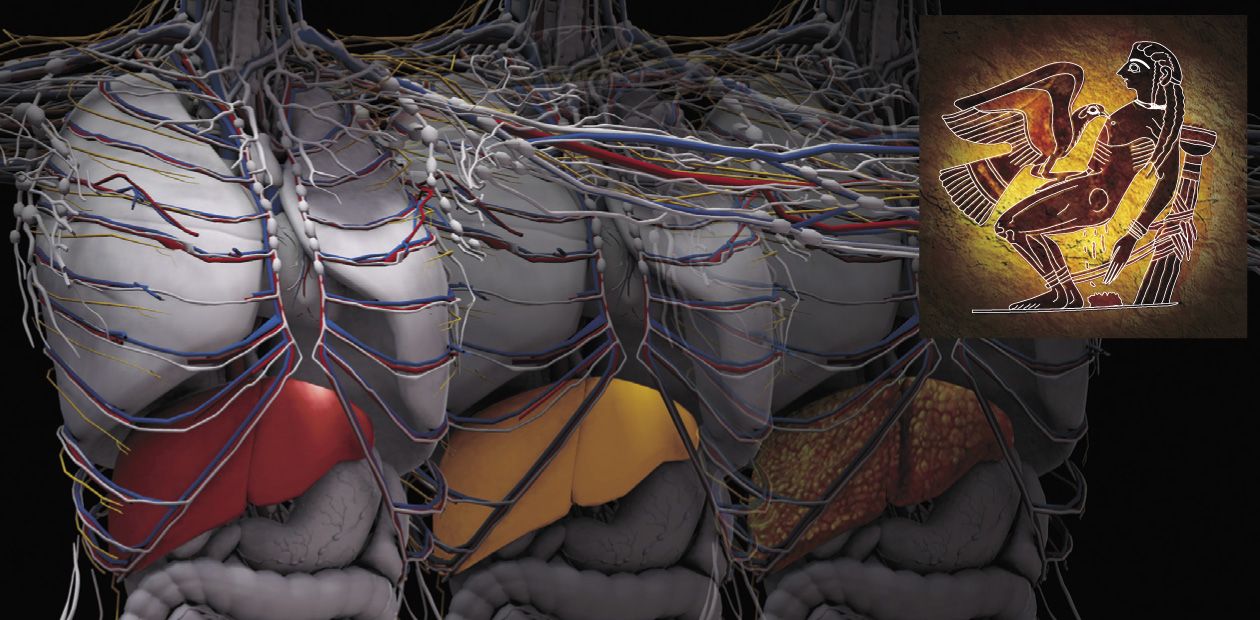
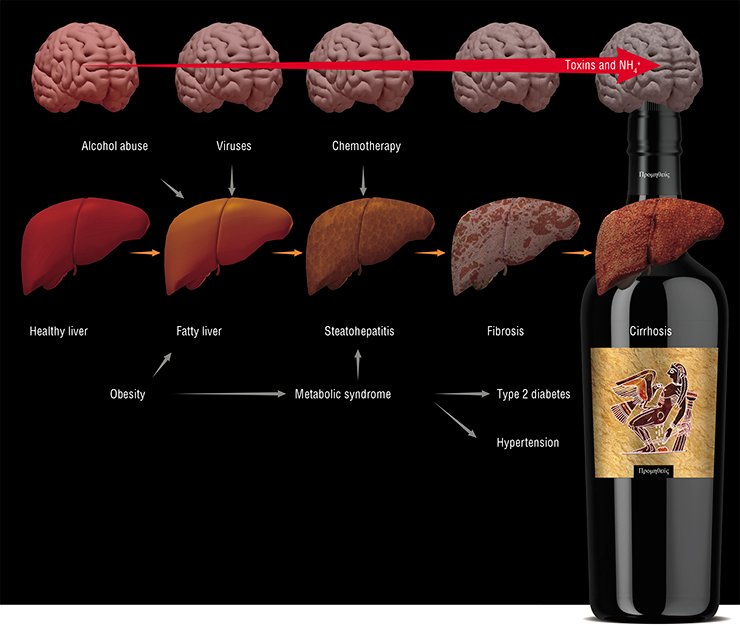
The main cause of chronic hepatic encephalopathy is progressive cirrhosis, i. e. irreversible replacement of normal liver tissue with fibrous (“scar”) connective tissue. Interestingly, the first person to notice the correlation between fatty degeneration of internal organs and the development of cardiovascular and liver diseases was René Laennec, a famous French physician and forensic pathologist, and inventor of the stethoscope. In 1819, he gave cirrhosis its name using the Greek word kirrhos for “tawny”, which indicated a change in the liver color caused by its fatty degeneration.
Cirrhosis can develop because of several illnesses, such as alcoholic or nonalcoholic (type 2 diabetes) steatohepatitis (an inflammatory process caused by fatty degeneration of the liver), viral hepatitides, and autoimmune diseases. The lifespan of progressive cirrhosis cases does not exceed 3–6 years. The annual mortality rate of such patients in Russia is from 75 000 to 100 000 individuals, although there are no official statistical data for this cohort.
Portal hypertension, i. e. hypertension in the hepatic portal system resulting from misperfusion and causing gastrointestinal bleedings, enlarged spleen, and so on, is among the most dangerous complications of cirrhosis along with chronic hepatic encephalopathy, which occur in 60 % of such patients. Progressing through successive stages of the disease, namely hepatitis, fibrosis, and cirrhosis, the liver gradually loses its function of an active filter. Excess ammonium and the corresponding secondary toxins (fatty acids, proinflammatory cytokines, neurosteroids, and others) influence the cells of the brain and of other organs, thereby inducing a slowly progressing generalized disease ending in a lethal outcome.
Acute hepatic encephalopathy develop when the liver loses its functions of an active filter over a short time span ranging from several hours to several days. Such an acute liver failure can be caused by viral, toxic, or drug impacts on the liver accompanied by its fatty degeneration. In some cases, this is associated with congenital defects of certain enzymes involved in different metabolic pathways, which leads to the accumulation of toxic metabolites that can cause liver dysfunction.
Of special importance are drug-induced liver injuries. About 1000 medicines of over 12 000 pharmaceuticals currently used in the world are potentially toxic when overdosed and, what is even more dangerous, in combination with other damaging factors. These substances include antibiotics, interferons, antiepileptics, antiinflammatory drugs, narcotic agents, and so on.
A typical example is the common aspirin, which has been in clinical practice since the late 19th century; it is now one of the 20 drugs in great demand. Administering aspirin against the background of some viral infections can cause rapid microdroplet fatty degeneration of the liver cells and of some other organs and tissues, with Reye’s syndrome and other similar diseases developing
Final collaborative work was published in 1896 (Nenski et al., 1896).This paper described for the first time the impact of ammonia on behavioral symptoms in healthy and shunted dogs and in animals with different types of digestion; ammonia distribution in different organs; and the implication of gut flora in this process.
Yet it took over half a century of studies to unambiguously correlate ‘‘the meat intoxication’’ syndrome in dogs with the human liver coma and regard the metabolism of ammonium in liver cirrhosis as the major cause of brain dysfunction (Burchi, 1927; Van Gulaert and Deviller, 1932; Sherlock et al., 1954).
It was determined that a drastic increase in the ammonium concentration in blood took place in septic shock, cardiac arrest, intensive physical exercise, and any oxygen deficiency (Parnas et al., 1926; Nelson et al., 1953). It was also discovered that some patients could develop encephalopathy without cirrhosis, for example, in fulminant fatty degeneration of the liver (Brain et al., 1927; Mann еt al., 1962; Reye et al., 1963).
Following the proverb that all new is well forgotten old, some researchers who were familiar with Pavlov’s works started to use ammonium salts for treating liver cirrhosis patients or for directly shunting the liver blood flow by surgery. Naturally, this led to lethal outcomes. Using a trial-and-error approach in treating patients with cirrhosis or hepatic encephalopathy, while lacking sufficient information about the mechanism and genesis of the pathological processes, was rather characteristic of the research in the first half of the 20th century and, unfortunately, was unavoidable charge for the acquired knowledge.
In the middle of the past century the term “hepatic coma” was replaced by the new term “porto- systemic encephalopathy” and later by the modern term “hepatic encephalopathy”, what emphasized the importance of the gut-liver-brain axis in the genesis of this syndrome.
First classifications of clinical diagnostic criteria for the clinical course of the patients with hepatic coma (Sherlock et al., 1954, 1961) and basic principles of the therapy (“a logical course of therapy”) (Walshe, 1953; Sherlock et al., 1954; McDermott, 1958) were formulated at that time and represent core elements of modern diagnostic criteria and treatment options of hepatic encephalopathy.
Finally, experimental models of hepatic encephalopathy involving laboratory animals were designed in the second half of the 20th century; they were used to study the mechanisms underlying the toxic effect of ammonium and of secondary toxins as well as to search for protective therapeutics. In the last quarter of the 20th century, the advent of intravital cell techniques involving fluorescence microscopy as well as immunochemical and genetic engineering methods shifted the research focus to subcellular and molecular levels.
Brain Edema: What are the Causes?
Hundreds of thousands of research papers on the mechanisms underlying the pathogenesis of hepatic encephalopathy and on search for the methods allowing for their correction have been published. Yet tens of the proposed theories reflect the research trends of the last decades rather than a deep insight into the problem.
In any case, there is no doubt that the development of hepatic encephalopathy is accompanied by an increase in the concentration of the key endotoxin, ammonium, in the blood and cells of body tissues as well as by growth in several secondary toxins, including proinflammatory cytokines (“cytokine storm”; Shawcross, 2012), long-chain fatty acids (Reye’s syndrome), neurosteroids, and so on. The stages of progression of encephalopathy follow the pattern from simple psychomotor disturbances to stupor, coma, and death.
It is currently believed that the progression of acute hepatic encephalopathy is mainly caused by the brain edema of a toxic or vasogenic (caused by an increase in the capillary permeability) nature. In other words, edema develops when the so-called toxic osmolytes glutamine, lactate, and alanine (the molecules whose accumulation in the cells leads to their swelling because of water inflow) accumulate in brain cells. This accumulation is a consequence of the dysfunction of neurons and astrocytes (“service” cells of the brain) or of increased permeability of the blood brain barrier caused by the dysfunction of vascular endothelium (internal lining cells) and of smooth muscle cells of blood vessels.
The brain edema, causing an increase in the intracranial pressure, is the major cause of coma progression and of subsequent death against the background of acute hepatic encephalopathy. However, the molecular mechanisms that underlie signaling dysfunctions of brain neural networks in comatose states are still uncertain.
The accumulation of ‘‘toxic osmolytes’’ is a compensatory response of different cell types to a drastic increase in the concentrations of ammonium and secondary toxins. However, the negative effect of the primary endotoxin goes beyond stimulating “toxic osmolytes” accumulation. As is known, ammonium and potassium have similar ionic radii. This allows ammonium to attack a number of cell cationic channels and various transporters, thereby radically disturbing ionic homeostasis in various organs and tissues. In addition, ammonium can negatively influence the signaling functions of neural networks affecting various types of cell receptors implicating still unknown mechanisms.
The list of cellular targets for ammonium and secondary toxins comprises several tens of items so far and is constantly growing. Correspondingly, there is a growing number of methods for treating hepatic encephalopathy; they utilize various blocking agents such as those inhibiting the synthesis of toxic, which can reduce the brain edema and weaken the lethal effect of toxins.
In chronic forms of hepatic encephalopathy, excess ammonium and secondary toxins can affect the cells of the brain and other organs and tissues leading to a slowly progressing multistage generalized disease with characteristic sets of metabolic and signaling alterations, and neuropsychic symptoms. The slow dying of such patients ends in a lethal outcome.
In this case the irreversibility of pathological processes in the cells of liver, brain, vascular system, and other organs and tissues is associated not only with the direct effect of toxins on the known cellular targets, but also with a change in the expression of key genes involved in various metabolic, transport, and signaling systems. However, the research of such mechanisms is still only on the agenda.
How is this to be Treated?
Surgery, i. e. donor liver transplantation, is currently the most effective method for coping with hepatic encephalopathies (Bajaj, 2010). It requires almost immediate (during the first 3–4 days) intervention in the case of acute states (Lee, 2003).
Reye’s syndrome is a rare yet life threatening acute state in children and teenagers; it can arise out of a viral infection (influenza, measles, or chickenpox) treated with drugs containing acetylsalicylic acid such as aspirin. The typical pattern of the disease is progression of liver fatty infiltration and rapid progressive encephalopathy caused by brain edema. The mechanisms underlying the effect of the entire set of toxins and liver fatty degeneration in such cases are still vague.In the absence of appropriate treatment, the patient’s state rapidly deteriorates to coma and respiratory arrest. The 1980s high mortality rate in the cohort of children treated with aspirin against the background of a viral infection made many developed countries prohibit aspirin as an antipyretic and antiinflammatory drug for children under 12 years old
Regretfully, the annual number of liver transplantations in Russia amounts to a weekly number of such surgeries in, for example, Spain. The surgical method of portal decompression proposed by Eck has not become widespread because of serious side effects. Vasoconstrictors (the substances that narrow the blood vessels and decrease the blood flow) and rubber band ligation of varicose vessels are now used for portal decompression.
Since the mid-20th century there have been many attempts to design efficient therapies for preventing the neurotoxic effects of ammonium. The currently available strategies for treating acute and chronic hepatic encephalopathies fall into two groups: first, the approaches directed towards decreasing the ammonium concentration in the blood (“Ammonia lowering strategies”) and, second, “Neuroprotective strategies”, or inhibitory strategies aimed at inhibiting the processes induced by ammonium and secondary toxins.
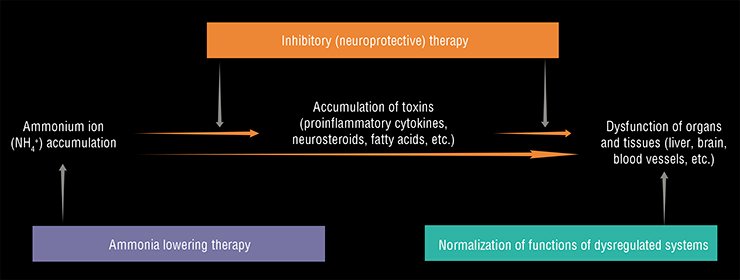
The methods for decreasing the concentration of ammonium in the blood, which were developed for the therapy of hepatic encephalopathy as early as the mid-20th century, are still topical and in great demand (McDermott, 1958; Al Sibae et al., 2009; Rose, 2012; Leise et al., 2014). These methods utilize the inhibition of ammonium production by intestinal bacteria and the activation of its conversion to urea in Krebs–Henseleit (urea) cycle in sick liver and to glutamine (an amino acid) in various organs and tissues.
To inhibit the activity of intestinal bacteria that produce ammonium, probiotics with a selective effect on intestinal microflora activity (for example, synthetic sugar isomers) or antibiotics (for example, neomycin) are used. Various combinations of amino and dicarboxylic acids can activate the reactions for endotoxin utilization. ‘‘Hepa-Merz’’ is an example of such a medicine; it activates the urea cycle. The main problems of this kind of therapy are the requirement of ongoing patients treatment by recommended pharmaceutical preparations (to escape their rebound effects (Jalan and Jalan, 2008); adverse side effects of antibiotics (O’Leary et al., 2015); and systemic impact of altered gut flora (Shawcross, 2015; Ahluwalia et al., 2016).
Until recently, the development of progressive liver cirrhosis was mainly associated with alcohol abuse and liver viral infections (hepatitides B, C, and E) in the patients over 40 years old. However, a drastic increase in the number of cirrhosis patients is expected in the coming years because of the increasing spread of obesity and type 2 diabetes. According to the WHO estimates, the current number of type 2 diabetes in Russia exceeds 16 million persons, children accounting for a quarter of this cohortAlong with these treatment options, hypothermia and injections of glucocorticoids, antiinflammatory agents, and other pharmaceuticals can be used for inpatients during resuscitation. Concomitant use of these practices in Europe and in the United States over the last two decades has led to a threefold reduction in lethal outcomes associated with brain edema in acute hepatic encephalopathies (Shawcross, 2012).
However, the great efforts of the last half century to design and use efficient neuroprotectors that can weaken or prevent the toxic effect of ammonium on the brain cells have failed (Braissant еt al., 2012). Such neuroprotective strategies utilize the inhibition of the key elements of signaling, transport, or metabolic systems in the brain that are excessively activated by increased ammonium concentrations.
One of such methods is the inhibition of glutamine synthase, the enzyme involved in the synthesis of “toxic osmolyte” glutamine (Schenker et al., 1967; Brusilow et al., 1986, 2012), and the blockade of glutamate NMDA-receptors (Felipo et al., 1996, 2013), the key excitatory receptors in the brain. The problem is that glutamine synthase acts in almost all organs and tissues, and glutamate receptors are also present in the cells of other tissues along with those in the brain. Therefore, these inhibitors administered to the whole body will naturally cause undesirable systemic side effects. Besides, the inhibitors of lactic acid synthesis (lactate) and of other “toxic” substances might cause similar consequences.
Initial studies at the Institute for Cell Biophysics, Institute for Theoretical and Experimental Biophysics, and Institute for Bioorganic Chemistry with the Russian Academy of Sciences (Pushchino, Moscow oblast) have demonstrated that ammonium hyperactivates neuronal networks, implicating several types of receptors and channels (Kononov et al., 2012, 2013, 2015; Dynnik et al., 2015). Besides, this toxic effect of ammonia may also be realized in the cells of the cardiovascular system (Averin AS et al., 2015) and , apparently, in all other electrically exitable cells.
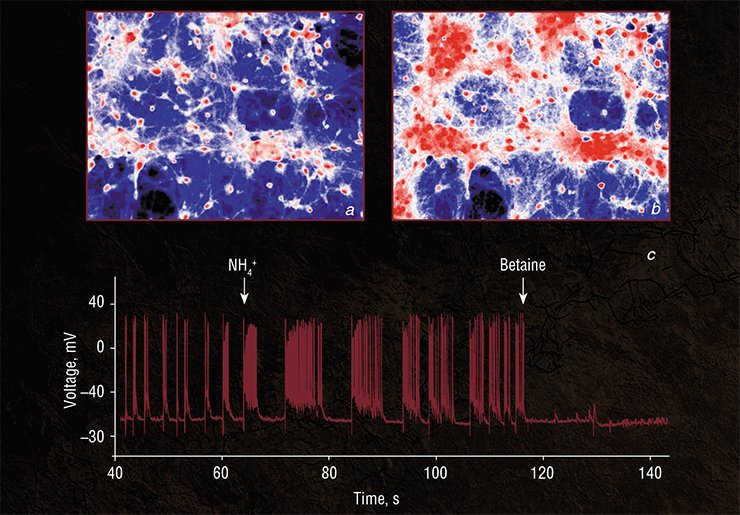
Thus, to diminish the negative impact of ammonia on various types of cells, it is not necessary to inhibit glutamine synthesis or to block excitatory glutamate NMDA-receptors.
Indeed, it seems meaningless to break the engine of a fast moving car if its brakes are in good working order. Today, we know a large class of substances that are able to decrease the hyperactivity of neuronal networks by activating the inhibitory receptors of brain neurons. Some of such substances, like betaine and L- carnitine, may combine several functions, being agonists of inhibitory receptors and true osmolytes, accelerating the removal of accumulated glutamine, alanine, lactate, etc. Moreover, the combinations of such substances can effectively reduce ammonium content in hyperammonemic animals (Tolmacheva et al., 2011; Grishina et al., 2015).
Our preliminary results indicate that complex compositions may have a better efficacy in the treatment of patients with encephalopathy (being addressed to several targets in all affected organs) than the efficacy of any single compound or of various couples due to the combined synergistic action of incorporated substances (having multiple functions) on deregulated metabolic, transport and signaling systems (Bogomolov et al., 2013; Dynnik et al., 2016). Moreover, such compositions do not have rebound effects. This means that permanent treatment of patients may be substituted by the periodical one.
Thus, although the history of research of hepatic encephalopathy spans 120 years, the current medical practice still relies on the inhibitory approach in treating the most severe consequences of liver failure. The objective cause here consists in a multifactorial and complex nature of the disease as well as in oversimplification of the structures and feedback regulatory mechanisms of corresponding metabolic and signaling systems. The current state of affairs is in many respects determined by the traditions in medical biochemistry and European pharmacology, based on monotherapy and inhibition of the processes triggered by toxins and pathogens rather than on restoring the functions of the affected systems.
Evidently, since there are no well examined and widely available cell technologies for efficient regeneration of the affected liver, the most promising approach now is the concurrent use of several therapeutic strategies focused on decreasing the content of toxins and activating deregulated metabolic and signaling cellular systems, and the regulatory systems of key genes.
Development of complex multi target compositions and using them in clinical practice will make it possible, figuratively speaking, to alleviate Prometheus’ suffering – that is, to extend the life and improve its quality for the patients with a severe diagnosis suffering of liver cirrhosis and hepatic encephalopathy. Certainly, only large integrated projects can rise to the challenges of such a scale.
References
Eck N. V., On ligation of the portal vein // Voen. Med. Zh. 1887. N. 130. P. 1–2 [in Russian].
Hahn M., Massen O., Nencki M., Pawlow J., Die Ecksche Fistel zwi der unteren Hohlvene und der Pfortader und ihre Folgen fur den Organismus // Arch. Exp.Pathol. Pharmakol. 1893. N. 32. P. 161—210.
Nencki M., Zaleski J. Ueber die Bestimmung des Ammoniaks in Thierischen Fluessigkeiten und Geweben // Arch. Exp. Pathol. Pharmakol. 1895. N. 36. P. 385—396.
Nencki M., Pawlow J. P., Zaleski J. Ueber den Ammoniakgehalt des Bluttes und der Organe. Die Harnstoffbildung bei den Saugetieren // Arch. Exp. Pathol. Pharmakol.1896. N. 37. P. 26—51.