"The Ob Disease"
“A human being as an environment”… This wording does not sound conventional, does it? Nonetheless, this is a well-known fact: our body is a sort of “microcosm”, a harbor for myriads of living beings, although of minute size. The majority of our lodgers are quite friendly: the microorganisms inhabiting our mucosae and gastrointestinal tract are good payers assisting us in digesting food, supplying us with vitamins, and so on. However, there are other relationships, along with the mutually beneficial interactions, which range from neutral to killing in its proper sense. Our “hero”—the liver fluke— is a parasite living at the expense of another organism (the host), causing damage to the host and yet not killing him at once
Historical Summary
Undoubtedly, the humans and our “hero” have known each other for a long time. In fact, it began when people started to consume freshwater fish from the water bodies that are natural incubators of this parasite. Yet the official acquaintance took place not so long ago: Karl Rudolphi, a German scientist, discovered this parasite in the bile ducts of the domestic cat at the beginning of the 19th century. However, our hero was not recognized at once—it was several times “re-discovered”, each time acquiring new names, until Rivolta, an Italian scientist, who also discovered this parasite in cats, recognized it as a separate species and named Distomum felineum. Since that time, the attributive “felineum” remained in its species name. However, it took only several years to find that, unfortunately, not only pets could be the hosts for this parasite.It was K. N. Vinogradov, a professor of Tomsk University, who did us the favor of discovering this regrettable fact by discovering the egg and parasite itself, an unknown fluke, in the gall and bile duct of a dead Siberian rafter. The parasite was “named” again, this time, Distomum sibiricum, i.e., Siberian fluke. Ever since, the instances of Siberian fluke infestation in humans, dogs, and cats become ever more frequent, first in Siberia and then in Europe. Note that the identity between the cat’s and dog’s liver fluke was found in the end of 19th century, and it was ascribed to the newly separated genus Opisthorchis. At this particular time, our hero got its present name—cat liver fluke (Opisthorchis felineus).
Further investigations of domestic parasitologists demonstrated that the geographic distribution of the disease caused by cat liver fluke practically coincided with the territory of the former Soviet Union. Correspondingly, in the early 1920s, Soviet scientists commenced a large-scale comprehensive study of this dangerous infectious agent. This study was conducted under the famous All-Union Helminthological Expeditions, initiated and guided by K. I. Skryabin, the most prominent Soviet parasitologist. Starting from 1919, these numerous expeditions examined from the parasitological situation the most remote region of this country. Opisthorchiasis was found in Donbass, North Dvina Province, and the Tobolsk North. The 70th Helminthological Expedition reported that “…the lower of the Ob River are the main opisthorchiasis nidus covering 100% of the human population as well as domestic and furry animals” (Skryabin, 1932).
Simultaneously with detecting the opisthorchiasis foci, parasitologists studied the biology of this parasite, the clinical signs and pathogenesis of the disease, and searched for the corresponding treatment methods. In the early 1930s, they discovered the liver fluke metacercariae in fish and in 1940, the expedition headed by N. N. Plotnikov, a well-known parasitologist, discovered the fluke larvae in Bithynia, small prosobranch gastropod mollusc. The mystery related to the liver fluke lifecycle began to unveil.
Plotnikov was the first to cure patient from liver fluke in the late 1940s. From mid-last century, they commenced paying much attention to the prevention of this parasitic disease, including development of appropriate technologies for fish processing and popularization of knowledge about the dangers of this disease. However, opisthorchiasis did not give in –– despite a close attention of physicians, veterinarians, and biologists. According to Plotnikov, an expert in this disease, heaps of dissertations on opisthorchiasis were defended, and yet it is still there. This is determined by many reasons: a wide geographical distribution of liver fluke, absence of reliable diagnostic and prevention tools, and gaps in our knowledge about the biology of the parasite itself and its hosts. Russia is still the leader in the prevalence of this most mass and dangerous helminthic disease.*
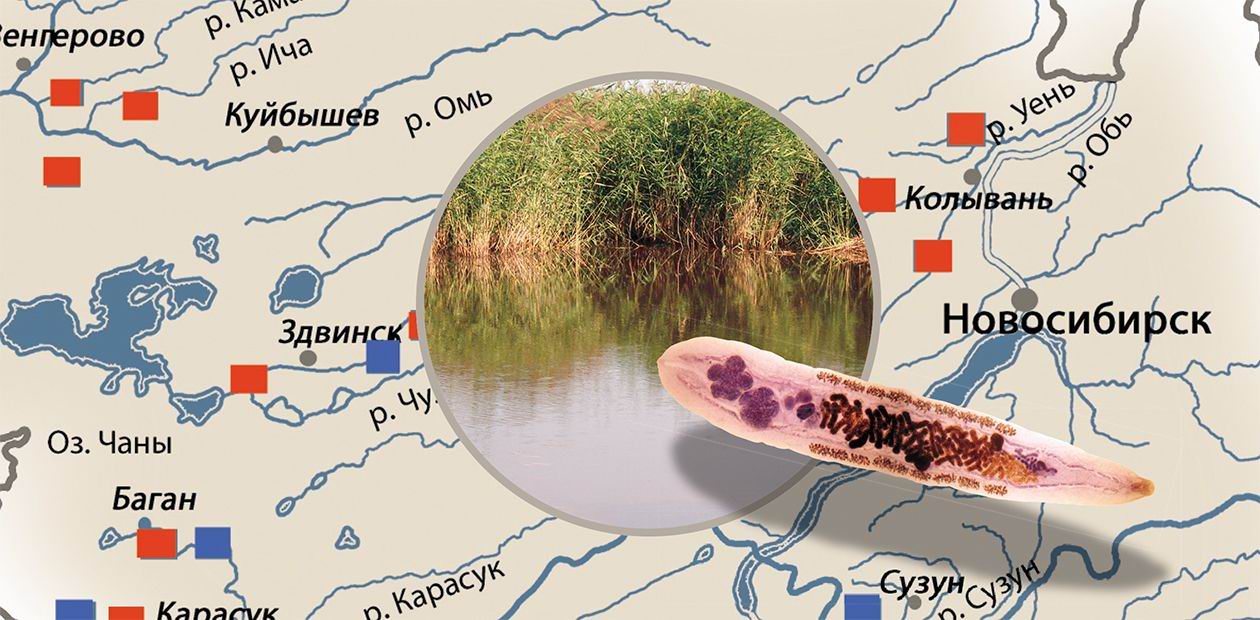
The world got acquainted with the disease, which we know as opisthorchiasis, 117 years ago; however, the Siberian population had known it much earlier: watchful Siberians called it “the Ob disease”. This name hit the mark: indeed the Ob opisthorchiasis focus is now the largest in Russia and the entire world. Thus, the population invasion rate in some sites of the Middle Ob region can reach 50—80% and even higher (Sidorov, 1985; Beer, 2005).
What is this worm, liver fluke?
From the standpoint of systematics, these organisms are trematodes or digenetic flukes, widespread parasitic flatworms. In this sense, the cat liver fluke (Opisthorchis felineus) is not alone: the large family of liver flukes contains 65 species belonging to 11 genera. The adult age parasitize in birds and mammals. Humans are among their hosts: over 21 million people worldwide are infested with liver flukes (Wiwanitkit, 2002). In addition to O. felineus, the most well-known of them are the civet liver fluke (O. viverrini) and Chinese liver fluke (Clonorchis sinensis). This so-called “liver fluke triad” causes similar animal and human diseases on the vast territory of Eurasia (Beer, 2005).
The natural area of cat liver fluke covers the territory from Western Europe to East Siberia, including Austria, Albania, Bulgaria, Hungary, Germany, Greece, Netherlands, Spain, Italy, Poland, Russia, Romania, Turkey, Finland, and France (Erchardt, 1962; Rotkiewicz, 1979; Schuster et al., 1988). The civet liver flukes are common in Southeast Asia (Laos, Thailand, Cambodia, and Vietnam) and the Chinese liver flukes, in China, Korea, Taiwan, northern part of Vietnam, Russian Far East, and Japan.
The disease caused by the liver fluke Opisthorchis felineus is found on the area comparable to the territory of the African continent. The population infestation rate at certain sites of the Ob focus, the largest in the world, can reach 90%The usual habitats of the “liver fluke triad” are planes located near rivers. The West Siberian Lowland, one of the largest lowlands on the globe, is very illustrative in this respect. Here, in the Ob—Irtysh river basin, the largest and most epidemiologically intensive nidus of this disease is located. This geography of liver flukes is not accidental but rather determined by their complex life cycle, implying a successive change of the hosts—intermediate, supplementary, and final. The main area of the most numerous, dangerous, and epidemiologically significant liver fluke species, O. felineus, is in West Siberia.
And the man is marching as the master…
The people that long ago inhabited the territory of West Siberia joined the parasitic system of opisthorchiasis agent, as one of the final hosts for the liver fluke. The sexually mature hermaphroditic individuals, maritae, parasitize humans. Humans share this dubious honor with several animals species, such as carnivores, insectivores, piscivores, and omnivores (totally, about 30 species).
Over a rather long life (10—12 years), these helminthes produce a tremendous number of small eggs (to half million annually!). With feces, the eggs enter the environment; and the lucky ones find themselves in the shallow freshwater of aqueous bodies, where they keep on the leaves and stones. The larva within the egg—miracidium—can stay alive for many months.
The next stage in the life of a liver fluke commences on the “convivial table” of the first intermediate host, Bithynia, small prosobranch gastropod mollusc belonging to the same genus. A larva from the egg ingested by a mollusc enters its body to undergo an amazing metamorphosis, starting to parthenogenesis (i. e., asexual reproduction)! This is a long and intricate process: from miracidium to sporocyst and from sporocyst to dozens of rediae. Each redia forms the so-called embryonic balls, which give rise to numerous tailed cercariae. Eventually, several thousand motile larvae, which then leave their host, are formed in one mollusc. Note that the developed larvae are as like as two peas: they are practically precise genetic copies of one another.
The moment when these microscopic live torpedoes realise into “the world “, i.e., into water, coincides with the hatching of various cyprinid species, the second intermediate hosts of this parasite. In the Novosibirsk oblast, the most frequent “prey” of cercariae is ide, roach, dace, bream, crucian carp, gudgeon, and small game-fish (belica and minnow, also sometimes eaten by the local population).
Actively penetrating into the fish subcutaneous tissue or muscles, cercariae becomes a metacercariae, sexually, adult, encyst; they discard their tails and encompass themselves with dense envelopes, turning into a certain thing, again resembling an initial egg (cyst). The cyst contains metacercaria, which proceeds through the stage of maturation. The number of cysts in individual fish can vary considerably, reaching tens of thousands in the case of a profound invasion; however, this does not affect much the health of the fish.
The last stage in the trematode life cycle is also connected with a “repast”; however, this time with the meal of the above mentioned final hosts. The cyst of metacercaria dissolves in the gastric and duodenal contents of a human or an animal that have eaten infested fish, and the young liver fluke migrates to the liver, which is its table and shelter. Reaching the liver, it clamps with its ventral sucker to the bile duct wall to commence a quiet and regular life. Being a hematophage, it feeds on the host blood, similar to mosquito females.
The adult liver flukes are hermaphrodites (have concurrently the female and male sexual organs). Their larvae partenogenetically (asexually) reproduce within the first intermediate host (mollusc)After 20—25 days, the liver fluke develops into a mature adult marita and finds itself a hermaphroditic pair. However, even without a pair (in the case of single invasion), the liver fluke produces numerous offspring as eggs with the miracidia inside. And some miracidia of this multitude will surely be lucky to find mollusc host…
It is evident from this lifecycle of the liver fluke that those fond of eating raw or insufficiently dried fish are to become the final hosts. Neither rediae nor cercariae, to say nothing about the eggs with miracidia, are dangerous for people.
Who are you, Mr. Liver Fluke?
Now let us return to our Siberian “showplace”—the largest in the world Ob opisthorchiasis nidus, located in the basin of the Ob and Irtysh Rivers on the territories of West Siberia and the Republic of Kazakhstan. Overall, eight liver fluke species have been discovered in West Siberia; mammals can be the final hosts for only three of them—O. felineus, M. bilis, and O. longissimus, whereas the remaining 5 species parasitize carnivores and aquatic birds (Karpenko, Fedorov, 1976; Fedorov, 1975, 1979; Yurlova, 1979). It was considered for a long time that only one species, O. felineus, was dangerous to humans, although many researchers admitted that other liver fluke species (first and foremost, Metorchis genus, belonging to the same family) could also infest humans (Skryabin, 1950; Fedorov et al., 1970; Sidorov, Belyakova, 1972).
This hypothesis had strong ecological grounds: as a rule, infested fish simultaneously contained the trematodes of various (two to three) species. Today it can be considered proved that, in addition to O. felineus, the disease named “opisthorchiasis” in West Siberia is also caused by the liver fluke Methorchis bilis. These two liver flukes similar in their structure and life style have the same range of second intermediate hosts, cyprinid fish. The main difference between O. felineus and M. bilis is the different first intermediate host. As a rule, the liver flukes are very specific in the first intermediate host range, and O. felineus is not an exception. However, M. bilis is not so specific and can parasitize two Bithynia species: species B. trosheli and B. tentaculata. This fact is very important as until recently the presence of infested first intermediate hosts, a certain mollusc species, in a water body was a criterion for detection of natural opisthorchiasis nidus.
Thus, we here approach the problem which is of extremely importance not only for research parasitologists, but also for the physicians treating opisthorchiasis. The fact is that a precise species identification of liver flukes is very difficult at all the stages of their complex life cycle; however, this is a keystone for both the helminthological studies in natural ecosystems and therapy of opisthorchiasis.
In particular, the species of the adult sexually mature individuals infesting humans is usually identified by an inefficient ovoscopic method, i.e., by the microscopic assay of the duodenal content or feces for the presence of liver fluke eggs. However, note that the liver fluke eggs are very difficult to identify not only at the level of species, but also of genus.
On the contrary, the adult individuals are characterized by a very high intraspecies variation in their morphological traits. Moreover, these differences are characteristic not only of the individuals from geographically distant sites, but also of the flukes infesting the hosts belonging to different species (for example, a marsh harrier and a crow); in addition, they are of different age!
When it is necessary to identify the species at a larval stage (for example, cercariae from molluscs), it is necessary to infest fish with cercariae, then to feed the fish infected with metacercariae to a final host, laboratory golden or dwarf hamsters. This stage is necessary, as the species identification based on morphological traits of metacercariae is also very difficult: characteristic of metacercariae is a pronounced age-related variation: in addition, the cyst sizes of different species overlap. It is evident that all these methods used for species identification can be accurate only when the diagnostician himself/herself has a long-term experience, a “good eye”.
The ovoscopic methods in liver flukes identification in humans have recently become even less efficient as the invasion intensity, i.e., the number of flukes per patient is universally decreasing (however, this in no way decreases the danger of the disease). It seems that a good alternative is the immunological methods, which are sensitive enough – diagnostics according to the fluke metabolic or degradation products inducing the immune response (specific antibodies) in the body. Many medical centers provide such service today.
Ide is among the Cyprinidae species most susceptible to liver fluke infestation. Large fish from the Ob River tributaries can contain up to several ten thousands of metacercariae, capable to infect those making a feast of fresh fishHowever, this approach is not free from shortcomings, as immunological methods can frequently give false positive responses in uninfected persons or patients with other parasites. This is explained by the fact that many helminthes as well as helminthes and their hosts have cross-reacting antigens, providing for the so-called molecular mimicry. For example, analysis of the O. felineus antigen structure has demonstrated that the blood sera of both infested and healthy individuals similarly react with the liver fluke proteins with a molecular weight of about 70 and 60 kDa**, i.e., the blood of both cohorts contains the corresponding antibodies. However, the blood serum of only opisthorchiasis patients react with the O. felineus protein of about 100 kDa (Glupov et al., 1997). This suggests that such studies can potentially give highly specific methods of opisthorchiasis diagnostics.
Some water bodies, such as the Malye Chany Lake and Kargat River (Novosibirsk oblast), contain all the components required for the life cycle of the opisthorchiasis agent. However, neither of these water bodies has foci of this disease. The reason for such resistance of intermediate hosts to the infestation is so far unknownThe advent of advanced precise molecular and information methods marked the new era in biology, including evolution, systematics, and ecology research. However, this refers to basic research; as for the problem of opisthorchiasis, it is not only biological and medical, but also social. Availability of the most precise diagnostic methods and most efficient and nontoxic drugs can alleviate the problem yet failing to solve it. Similar to tick-borne encephalitis, opisthorchiasis is a natural nidal disease, i.e., it has a long evolutionary history and stable biocenotic grounds. The involvement of humans in these natural processes is an inevitable consequence of colonization of uninhabited Siberian lands. Our current knowledge about the pathogen itself and its natural circulation is sufficient to use it for avoiding the unenviable role of a link in the transmission of this parasite.
References
Beer S. A. The Biology of Opisthorchiasis Agent—Moscow: KMK Press, 2005.
Fedorov K. P. The liver fluke ecology in the Novosibirsk oblast // The Ecology and Morphology of Helminthes in Western Siberia.—Novosibirsk: Nauka, 1979.—Pp. 5—55.
Fedorov K. P., Belov G. F., Naumov V. A., Khokhlova N. G. The problem of liver fluke infestations in West Siberia // The Parasites and Parasitic Diseases in West Siberia.—Novosibirsk, 1996.—Pp. 96—99.
Fedorov K. P., Naumov V. A., Kuznetsov V. G. On several topical issues of the problem of human and animals liver fluke diseases // Med. Parazitol. Parazit. Bolezni.—2002.—No. 3.
Glupov V. V., Khokhlova N. I., Khvoshchevskaya M. F., et al. Use of immunoblotting in studying Opisthorchis felineus (Rivolta, 1884) antigens. // Med. Parazitol.—1997.—No. 1. —Pp. 17—19.
Serbina E. N., Yurlova N. I. Involvement of Codiella troscheli (Mollusca: Prosobranchia) in the lifecycle of Metorchis albidus (Trematoda: Opisthorchidae) // Med. Parazitol.—2002.—No. 3. —Pp. 21—23.
Sous’ S. M., Rostovtsev A. A. The Fish Parasites of the Novosibirsk Oblast, Part 1, Tyumen, 2006.
The author and editorial body are grateful to Dr. V. V. Glupov, Dr. V. D. Gulyaev, Dr. E. N. Serbina, Dr. A. I. Chechulin, and A. V. Krivopalov for their assistance in preparing the manuscript and the artwork.
* According to Beer, 2005.
** kDa, kilodalton, a unit of protein molecular weight