Notes from Personal File of Liver Flukes
For a long time, the agent of opisthorchiasis, a widespread parasitic disease caused by eating infected fish, was mainly the object of medical and parasitological studies. However, state-of-the-art molecular genetic methods in combination with bioinformatics approaches can provide fundamentally new data on the biology and structure–function organization of the genome of this parasite, including details of the molecular principles underlying its pathogenicity and carcinogenic activity. This opens new vistas for prevention and treatment of opisthorchiasis
For a long time, the agent of opisthorchiasis, a widespread parasitic disease caused by eating infected fish, was mainly the object of medical and parasitological studies. However, state-of-the-art molecular genetic methods in combination with bioinformatics approaches can provide fundamentally new data on the biology and structure–function organization of the genome of this parasite, including details of the molecular principles underlying its pathogenicity and carcinogenic activity. This opens new vistas for prevention and treatment of opisthorchiasis
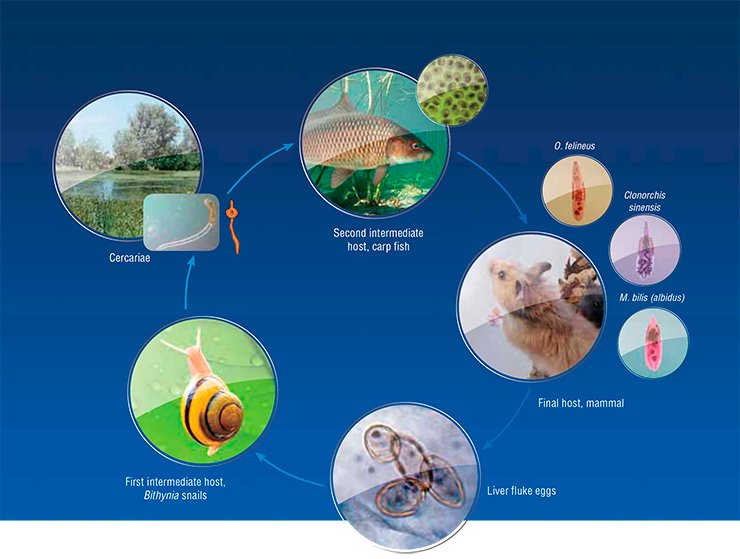
Opisthorchiasis is a severe focal parasitic disease caused by the liver fluke. These parasitic trematodes (or flatworms) belong to the family Opishtorchiidae. The interest to opisthorchiasis is not accidental, being dictated by a high social significance of this disease in Russia, especially, in Western Siberia.
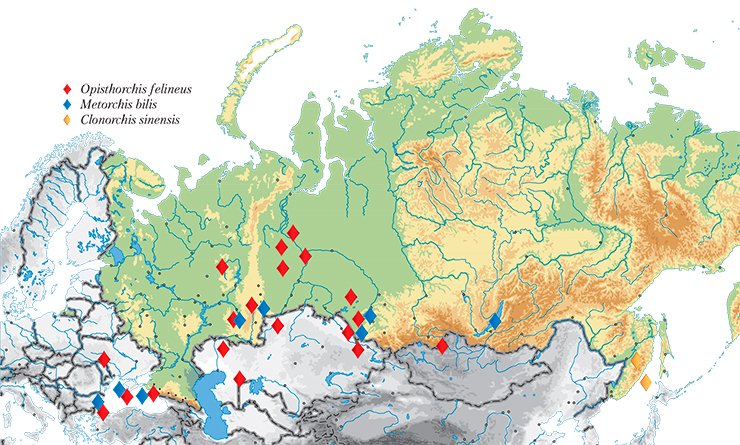
The largest natural focus of opisthorchiasis was discovered there in the Ob–Irtysh River basin as early as 1929. Unfortunately, the epidemiological situation over the elapsed decades has not improved at all: according to the Rospotrebnadzor (Russian Federal Service on Surveillance for Consumer Rights Protection and Human Welfare) data as of 2010, up to 90—95% of the population in the lower reaches of the Irtysh River and middle reaches of the Ob River were infested with the liver fluke, including preschool children. The morbidity rates for opisthorchiasis in different localities of this focus exceed threefold to 28-fold the average level for Russia (On the Sanitary and Epidemiological Situation..., 2008).
A systematic study of opisthorchiasis prevalence in Russia started as early as the 1920s. Over 40 years, the All-Union Helminthological Expeditions, initiated and supported by Academician K.I. Skryabin, have explored almost all area of the Soviet Union. Thus, they found that the distribution range of the main opisthorchiasis agent, Opisthorchis felineus (feline or Siberian fluke), in this country is vast and spans from the Biryusa River in the east to the western borders of the countryIn addition to the Ob–Irtysh River basin, the opisthorchiasis area of concern includes Kazakhstan as well as Eastern European and Southeast Asian countries. Moreover, liver flukes are occasionally encountered in the areas previously free of this parasite: the liver fluke expands, “conquering” ever new regions.
Taking into account the broad geography of liver fluke distribution, of doubtless interest to specialists is to find out whether the separate populations of this parasite in different parts of its distribution range display characteristic genetic distinctions. These studies, purely theoretical from the first glance, have an important applied perspective, since the genetic differences between parasites are the particular factors that determine significant medical characteristics, such as diversity in clinical manifestations, probability for emergence of drug resistance, and different immunogenicity (the ability to induce production of specific antibodies) of the parasites. All these data are important for prediction of the disease course and induction of the immune response in infested individuals as well as for elaboration of diagnostic tools and vaccines.
Since 2005, the Institute of Cytology and Genetics (Novosibirsk) is involved in such an integrated research into opisthorchiasis and its pathogens. Analysis of the population genetic structure of these helminths started from creation of a unique collection comprising the liver flukes from different geographic localities, namely, Western Siberia, European Russia, and Northern Kazakhstan. So far, this collection contains about 300 specimens of Opisthorchis felineus, the major opisthorchiasis agent in this country, and about 300 specimens of other Opishtorchiidae species, including those of epidemiological importance.
Method as a basis
The genetic diversity of helminths from different habitats has been assessed by comparative analysis of genomic markers, namely, DNA fragments. This method, widely used in current molecular biology, allows for a reliable estimation of the degree of kinship for both individuals and their groups. Therefore, it is used for determining closely related species and reconstructing their evolutionary history.
Typically, the single nucleotide polymorphisms (that is, single “letters” of the genetic code differing in the compared DNA sequences) in a certain selected DNA region rather than the whole region are used as such markers. The rate of such single nucleotide substitutions (mutations) that appear due to random reasons is very low, amounting to approximately 10–8–10–12 per cell per generation. That is why the genome regions with a high rate of mutation accumulation are selected as genomic markers. This is characteristic of the genome regions that for some reasons or other escape the natural selection, that is, are selectively neutral.
The nuclear DNA sequences not coding for any proteins or the DNA of cell organelles, mitochondria, meet this requirement. The latter is inherited in a strictly maternal manner with the egg cell cytoplasm and is not involved in recombination (exchange of chromosome regions during cell division). The accumulation rate for neutral mutations in mitochondrial genes is five–tenfold higher as compared with the genes encoded in the nuclear DNA.
It is commonly believed that the number of accumulated single nucleotide substitutions in selectively neutral DNA regions displays linear time dependence. Correspondingly, a mere counting of these substitutions may give an approximate dating of evolutionary events.
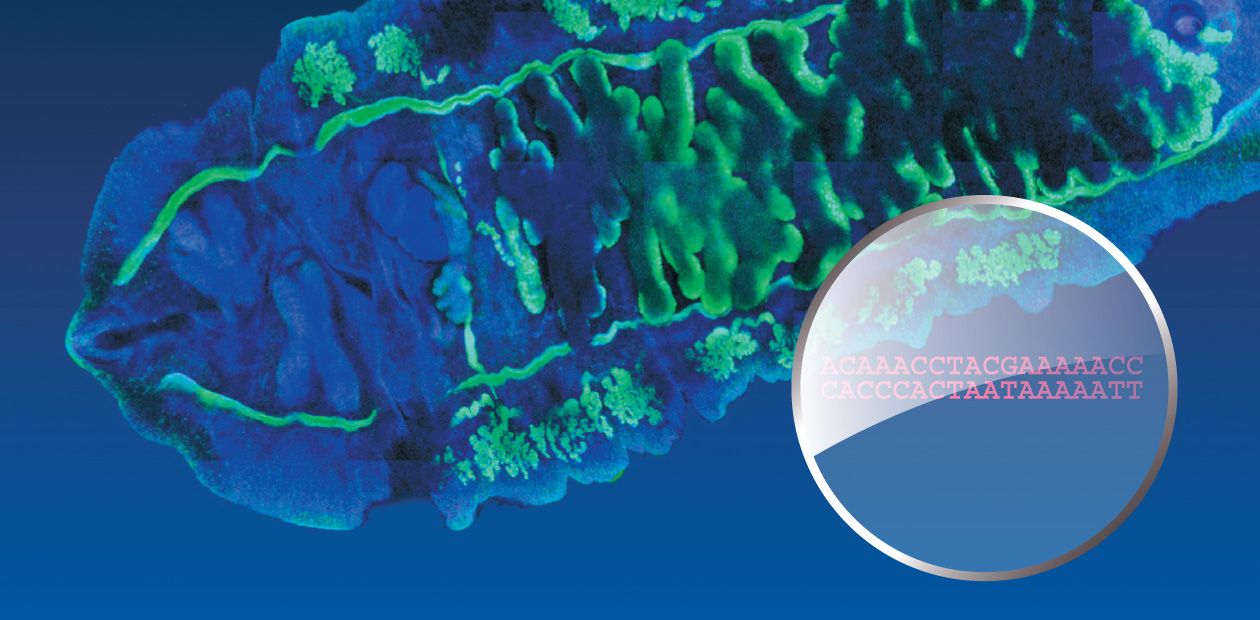
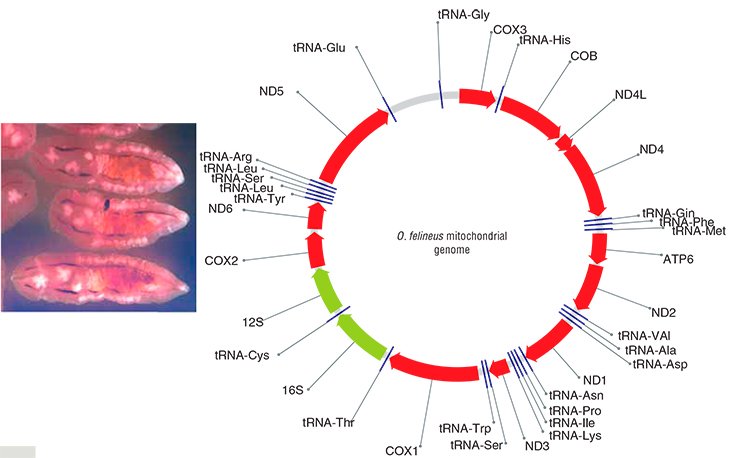
In order to find species-specific genome markers, it is first necessary to sequence a genome (that is, to “read” its nucleotide sequence). Correspondingly, the initial research task was to sequence some fragments of the nuclear DNA and the complete mitochondrial genome of O. felineus, which was successfully done at the Institute of Cytology and Genetics under an integrated project on liver flukes.
Thus, eight suitable genetic markers were detected and three of them (two mitochondrial and one nuclear) were selected for genotyping the collection specimens of liver flukes. Note also that although analogous markers have been recently used in population and phylogeographic studies of other parasitic trematodes in Europe, East and Southeast Asia, Africa, and America, O. felineus was left beyond this research.
A “bottleneck” of the Ice Age
The genome markers designed at the Institute of Cytology and Genetics have allowed for “sorting out of the mess” in the family of liver flukes, which, in particular, contains two closely related species—the above mentioned O. felineus and Clonorchis sinensis, causing clonorchiasis, the disease very similar to opisthorchiasis. The analysis demonstrates that these two species diverged from a common ancestor approximately three million years ago.
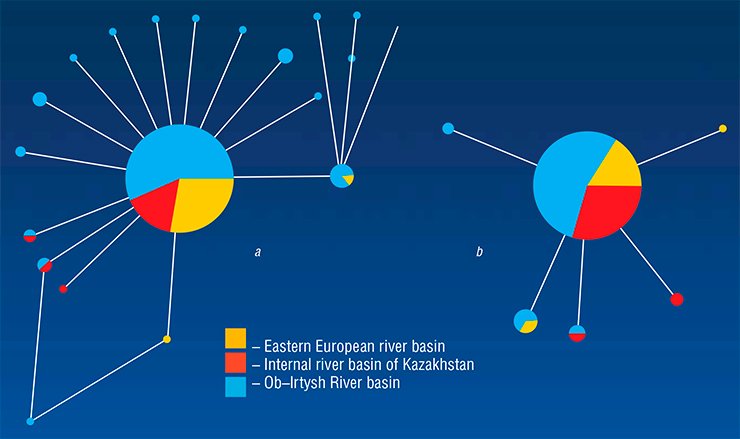
The subsequent evolutionary history of O. felineus seems rather dramatic. The fact is that three liver fluke subspecies were earlier hypothesized, namely, Siberian, Kazakhstan, and Eastern European, correspondingly inhabiting the Ob–Irtysh and Yenisei, Nura–Sarysuk, and Volga, Don, and Ural River basins (Beer, 2005). Therefore, it was expected that the collection specimens from so geographically distant populations would display noticeable genetic distinctions, since the ecological conditions were also drastically different. However, despite these assumptions, the examined liver fluke specimens displayed a very low genetic diversity unlike the earlier studied trematode species (Brusentsov et al., unpublished data).
What is the reason of such an amazing genetic uniformity of liver flukes? Presumably, this uniformity is a result of drastic reduction in the size of the only ancestral O. felineus population in Eurasia that survived after the Pleistocene glaciations. These glaciations were accompanied by a reduction in the habitats of liver fluke intermediate hosts (freshwater mollusks and fish) as well as in their infestation rates, which at that time did not exceed 2% (Beer, 2005). This phenomenon—when population passes through the stage of a critically low size – is referred to as the “bottleneck”; as a rule, a bottleneck results in a drastic impoverishment of the population gene pool.
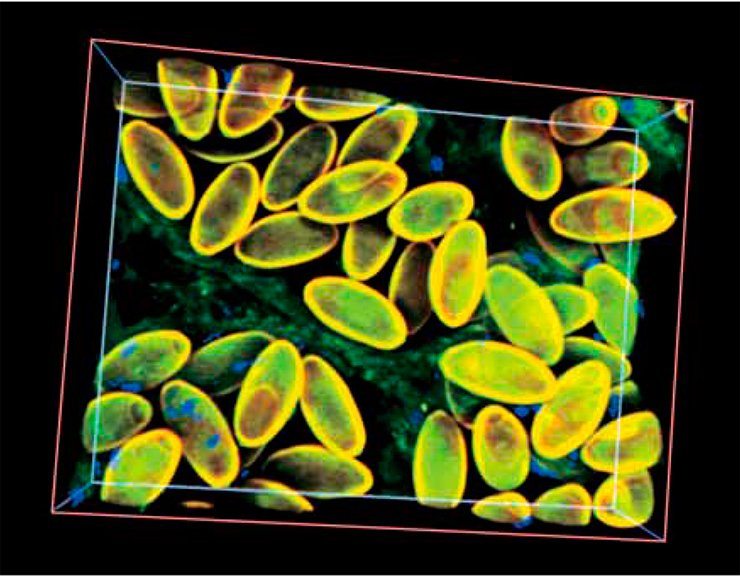
With further warming and development of new river basins, liver flukes restored the size of their ancestral population. According to the estimations, the period of population explosion in the history of this species started 21,000—25,000 years ago (and continues until now); presumably, a facultative hermaphrodite reproduction of the liver fluke significantly enhanced this explosion.
Note that the above mentioned trematode species other than O. felineus, which are also able to reproduce in a hermaphrodite manner, still display considerably higher genetic diversity. However, a relatively “poor” O. felineus gene pool has not prevented this species from restoring and successfully expanding its distribution range.
Unfortunately, the conducted study does not allow the location of ancestral liver fluke population to be precisely determined and its dispersal routes to be traced. Nonetheless, taking into account an intricate O. felineus life cycle, it looks quite evident that the direction and rate of its expansion have been determined by the migratory capacities of its hosts. The fact that opisthorchiasis is currently prevalent only in certain geographic localities is in many respects determined by the environmental preferences of O. felineus first intermediate host – the freshwater mollusk Bithynia, which dies in seawater. However, human, having almost no geographic barriers, is the definitive host of liver flukes.
In one tube
Genetic markers also have allowed a topical medical problem of a precise species identification of liver flukes to be solved. The fact is that along with O. felineus, another epidemiologically significant species, Metorchis bilis, circulates in Russia, Kazakhstan, and Western European countries. As for the Far East, additional trematode species C. sinensis, common for Southeast Asian countries, is found there.
All these trematodes cause diseases with very similar clinical manifestations, interfering with precise diagnosis based on the disease symptoms alone. Microscopic examination of the eggs of these helminths also fails to distinguish between the species due to their similarity, so that the diagnosis based on testing the fecal or duodenal samples depends on the qualification of laboratory assistant. The situation is aggravated by the possibility of mixed infection.
Today, PCR diagnostics of the diseases caused by trematodes gives the most precise results. PCR-based test kits have been designed abroad since the 1990s. In this country, the start was delayed; however, PCR diagnostic kits for opisthorchiasis have been actively elaborated in Russia, in particular, at the Institute of Cytology and Genetics. These diagnostic kits are able to detect genetic fragments strictly specific of a particular pathogen species in any laboratory samples. Since this method allows genetic markers for several helminths to be simultaneously used, one test is sufficient for a precise identification of parasitic agents.
Species identification of a parasite is of great importance. For example, the infection source and site can be thus determined. But the most important thing is that the agents of opisthorchiasis, metorchiasis, and clonorchiasis differ in a number of biological characteristics, which may influence the disease course and prognosis, potential complications, and the degree of parasite’s sensitivity to drugs.
In particular, it has been experimentally demonstrated in golden hamster model that two liver fluke species, O. felineus and O. viverrini, both met in Southeast Asian countries, differ in the degree of their attack on the host organism (Lvova et al., 2012). By the way, another trematode similar to O. viverrini, Haplorchis taichui, is widespread in Southeast Asia; however, infection with this trematode does not lead to a severe disease (Lovis et al., 2009). This example shows how important is to differentially diagnose trematodiases for an adequate treatment prescription.
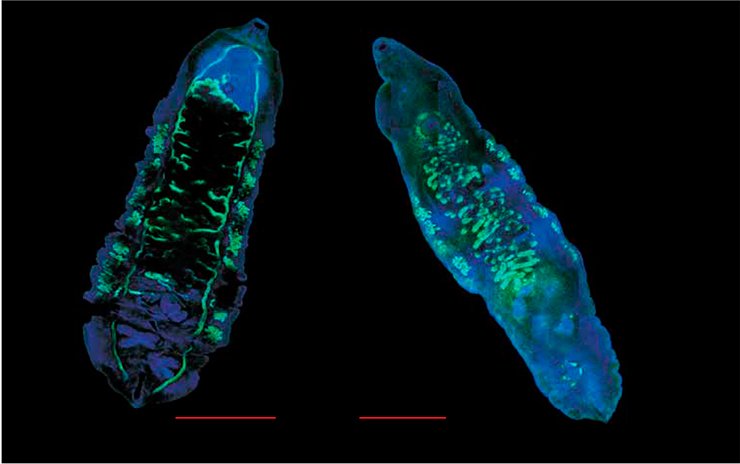
In addition, a chronic trematode invasion can lead to serious health complications even at a low infection rate. However, physicians in such cases are unable to detect liver fluke eggs in laboratory samples using common light microscopy examination, which frequently leads to postmortem diagnosis (Müller et al., 2007). As for PCR-based diagnostics, this is a much more sensitive method for such cases.
Thus, despite its dramatic evolutionary history, the parasitic trematode O. felineus not only succeeded in surviving, but also, despite the loss of part of its gene pool, is a thriving species with high adaptation potential, including high invasiveness, i.e., the capacity of invading the host body.
The test kits for PCR-based diagnostics of opisthorchiasis designed by Novosibirsk scientists have successfully passed laboratory trials, and their testing with clinical samples is close to completion. These diagnostic kits already allowed mixed trematode invasions to be detected in a group of patients (Brusentsov et al., 2010). This evidently demonstrates that such diagnostic tests should be included into the toolkit for laboratory diagnostics of opisthorchiasis. Production technology for these diagnostic kits is under development.
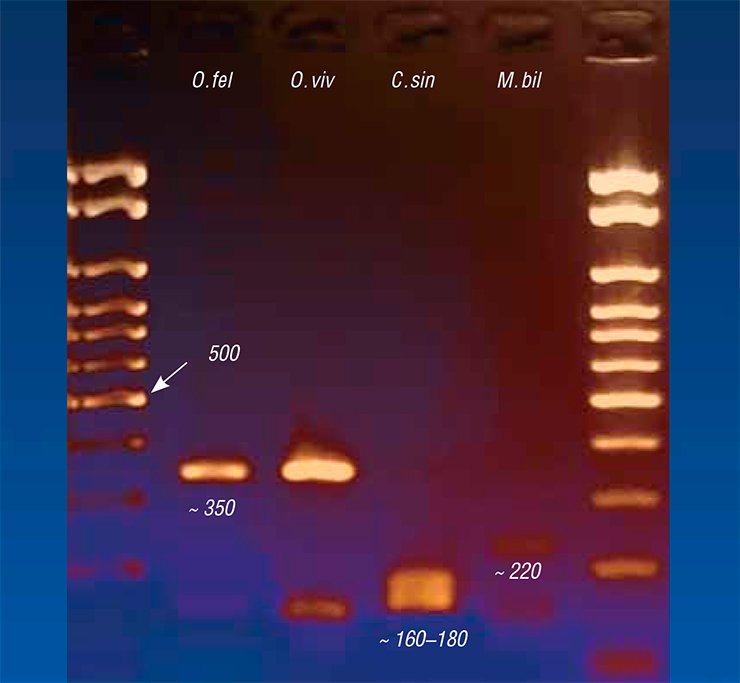
Nonetheless, the DNA-based diagnostics for helminthiases have not yet become a routine medical practice. Currently, the most widespread is serological diagnostics, in particular, enzyme immunoassay (EIA), and a number of test kits are commercially produced for this purpose. This approach is based on detection of the antibodies specific to antigens of the parasite in the patient’s blood serum. A flaw of EIA is its poor sensitivity and specificity: it rather fails when the antibody concentration is low and may give a false positive result if the diagnostic antigen reacts with a molecular target other than the “necessary” antibodies.
The accuracy of EIA may be increased by cloning the genes encoding the antigens specific of the liver fluke. Such genetically engineered antigenic proteins of O. felineus suitable for designing new generation diagnostic test kits have been obtained at the Institute of Cytology and Genetics. They may be used for detection of most minute amounts of specific antibodies to the parasite in the blood serum. However, it takes time for these diagnostic kits to be widely used in medical practice.
Undoubtedly, introduction of novel medical technologies will influence the treatment quality and reduce the rehabilitation period. There are many examples of this kind, and most illustrative of them are associated with diagnostics of bacterial infections, which passed from petri dishes to PCR tubes. However, the novel technologies do not annul the traditional assays but rather considerably increase the sensitivity and accuracy of tests, especially for complex clinical cases.
Expert’s Comment Integrated molecular genetic studies of opisthorchiasis and its agents conducted by Novosibirsk scientists (with participation of research teams from other cities) is a large and very serious project, which includes the expensive and ambitious component–genome sequencing of the liver fluke, the agent of this parasitic disease in this country.Note that creation of a new diagnostic tool is just only one applied outcome of this project out of many potential ones. The knowledge about molecular genetic diversity of this parasite yielded by this project will further form the background for detecting the therapeutic targets for opisthorchiasis treatment, which is no less important issue than its precise diagnosis. Note here that the only drug, praziquantel, almost exclusively used for the chemotherapy of opisthorchiasis, has several negative side effects.
On the other hand, we should not underestimate the significance of this new highly specific and sensitive method allowing for a precise identification of the pathogen and correspondingly, for prescription of timely and adequate treatment. The method currently used for diagnostics of opisthorchiasis (light microscopy assay of fecal samples for helminth eggs) requires highly qualified personnel, which is rather in demand. DNA diagnostics requires more complex equipment but considerably decreases the human factor aspect. A wide use of DNA diagnostics requires DNA isolation from clinical material (fecal samples), and this problem was also solved under this project.
Indeed, EIA allows for detection of the antibodies to the liver fluke. However, these two methods, PCR and EIA, should not be opposed but rather regarded as counterparts of the diagnostic process. EIA is more appropriate for controlling the treatment via monitoring activity of the immune system, while PCR is a better tool for screening and primary detection of disease.
As for the availability of such a precise diagnostic tool, its importance is determined by the fact that opisthorchiasis is frequently asymptomatic. Moreover, it has been proved that one of the most severe complications of opisthorchiasis is cholangiocarcinoma, malignant tumor of bile ducts, which suggests that the population of endemic foci of opisthorchiasis should be examined for liver fluke invasion on a regular bases and correspondingly treated when the disease is detected.
Siberian State Medical University (Tomsk)
References
Yurlova N. I. The Ob Disease // Science First Hand. 2008. No 1 (19). P. 12—22.
Kolchanov N. A., Mordvinov V. A. Parasitosis from A to T // Science First Hand. 2008. No. 1 (19). P 22—36.
Pal’tsev A. I. Sistemnomu zabolevaniyu – sistemnyi podkhod // Nauka iz pervykh ruk. 2008. №2 (20). C. 22—27.
Beer S. A. Biologiya vozbuditelya opistorkhoza. KMK, 2005. 336 s.
Brusentsov I. I., Katokhin A. V., Sakharovskaya Z. V. i dr. DNK-diagnostika mikst-invazii Opisthorchis felineus i Metorchis bilis s pomoshch’yu metoda PTsR // Meditsinskaya parazitologiya i parazitarnye bolezni. 2010. № 2. S.10—13.
Menyavtseva T. A., Ratner G. M., Struchkova S. V. i dr. Immunofermentnyi analiz v diagnostike opistorkhoza. Soobshchenie 1. Razrabotka immunofermentnogo metoda opredeleniya IgM - antitel k opistorkhoznomu antigenu // Meditsinskaya parazitologiya i parazitarnye bolezni. 1996. № 1. S. 41—43.