Big CHALLENGES generate BIG people
The author’s statement which is the headline of this contribution may seem as controversial as the notorious chicken and egg problem. The most important of all, however, must be bringing these two things together. It is there, at these meeting points, that history is made. An illustrative example is the Akademgorodok of Novosibirsk, a backwater town that has turned into a forefront of super activists striving to push forward the frontiers of knowledge and make the world around them better
I first came to Akademgorodok in January 1977, as a “travel companion” of Kirill Ilyich Zamaraev, who later became an Academician and Director (after G. K. Boreskov) of the Institute of Catalysis SB RAS. It was bitterly cold, and Kirill Ilyich got his face frostbitten when we went skiing at –26 °C. Till his dying day, he had the habit of rubbing the frostbitten cheek.

I am from Minsk, and by that time I had graduated from the Moscow Institute of Physics and Technology (MIPT). In those years, the new research areas, bionics and biophysics, were very popular and I was dreaming to become a biophysicist. The MIPT had an appropriate department but the meetings of its professors with undergraduates seemed so boring that I decided to transfer to the Biology Department of Moscow State University. I studied up on all the textbooks for the first two years and went to the Dean’s Office to take back my documents but they did not give them to me…As a result, I had long-doubts about my future occupation until the upper-year students advised me to decide on the director of diploma first and called my attention to K. I. Zamaraev, who gave us workshops on chemical kinetics in English.
“I am as much interested in technical and engineering problems as in basic research. This is attributed to my backstory. At the time of Khrushchev, polytechnic education was introduced in high school, and twice a week we worked at a factory. After three years’ experience as a metalworker at the Minsk Automobile Factory, I was straightaway awarded the third skill-category out of the five existing. In addition, having worked in a student construction brigade, I officially obtained a carpenter’s skill grade and a glassblower’s grade during my studies in the Moscow Institute of Physics and Technology. As a result, when I moved to Akademgorodok, I happened to be one of the few people in the Institute of Catalysis sharing a common language with the workshop supervisor.”By the way, the MIPT, which at the time was considered to be on a par with Harvard, offered an unconventional education: a comprehensive training in mathematics, physics, chemistry and technology plus up to four languages including Japanese. I graduated as an engineer-physicist specializing in the chemistry of high-speed processes, and I was eager to work in any area. It was Zamaraev, who was not only an brilliant experimentalist in physical chemistry but also a remarkable personality literally catching the people around him with his ideas and way of life, who to a great extent defined my future. Later, he became a co-supervisor of my postgraduate thesis at the Moscow Institute of Chemical Physics together with G. M. Zhidomirov, a specialist in quantum chemistry.
It is little wonder that when Kirill Ilyich invited me (after I had defended my Candidate’s thesis) to go to Novosibirsk with him, where he had been several times as a postgraduate of V. V. Voevodsky), I readily agreed. By that time, I had taken up a new topic – the use of solar power through chemical processes. The Akademgorodok of Novosibirsk offered much better opportunities for these studies than our Moscow institute of ripe age with tough competition for diploma students. When I made the decision to leave Moscow, many of my friends twisted a finger at a temple: in those years, the Moscow registration that I got as a junior researcher of a research institute was hard currency…
We finally moved to Akademgorodok in the May of 1977, with a galaxy of eight high-achieving MIPT graduates, mostly “recruited” by me. Now, forty years later, I feel so happy that I had left Moscow.
Town of super activists
In the capital, my office was near the Leninsky Prospekt, which was a regular route of foreign delegations, and we were often drawn away from our work and asked to greet them by waving flags. In Novosibirsk, you could just do work and relax when you felt like it. The matter is that the Siberian Branch had united not mere amateurs of science but those for whom science came first. All those people had left their comfy old haunts and came to Siberia with the express aim to do science.

According to a classic of history, Lev N. Gumilev, any country and society go through several development stages. One of them, the most interesting, is “passionary.” It is marked by the emergence of a great number of people with an irresistible achievement drive and hands-on approach to altering life for the better. When we arrived at Akademgorodok, it was at the peak of passionarity; the Siberian Branch of the USSR Academy of Sciences was twenty years old. Afterwards, to be honest, this attitude began to wane for different reasons but I do hope it is going to return some time later: according to that same Gumilev, all processes are cyclic.
When we were young, we would often leave the institute after ten p. m., and the lights in all the rooms were still on – now, you can see lights in only two or three windows. Big changes have taken place not in the quality of specialists (I think it is even better now) but in the personal interests and driving force. The inner spring that holds you is different now. And I’m happy that my spring was made back then.

…The first months after our arrival we stuck together. It was not an easy time because, though catalysis was a buzz word, the MIPT did not virtually deal with it. The good thing was that we were free to search, did not have to fill in many papers and had an opportunity to receive the equipment needed. My team within the laboratory led by Zamaraev was mostly involved in research into the artificial reproduction of natural photosynthesis. At that time, nobody knew how to do it. The very word “photocatalysis” was not taken seriously whereas today almost a third of all publications is dedicated to catalysis.
I believe that the most interesting thing for a professional is to develop an entirely new topic, when you do not depend on equipment or research techniques. We had quite an achievable aim: if nature has created plants, people can create functional analogs of living systems. And so it was. Moreover, there are now four companies in Russia that make special-purpose appliances based on photocatalysis for cleaning indoor air.
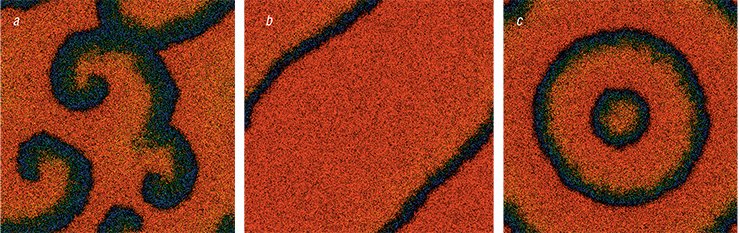
It is known that the so-called critical phenomena may arise in catalytic systems under certain conditions. These phenomena include:
hysteresis, a process with an ambiguous dependence of the reaction rate on the external factors, e. g. temperature or pressure; and
auto-oscillations, periodic changes of the reaction rate and reagent composition on the catalyst surface in time under constant external parameters – pressure, temperature and gas leakage. Under certain conditions, even more spectacular phenomena can be observed in the autooscillation mode. These are periodic in time and space dissipative structures, also called chemical waves. Investigation of these phenomena provides a deeper insight into the mechanisms of chemical reactions.
As a rule, chemists attribute oscillations during CO oxidation on palladium group metals to the formation of two forms of oxygen: active and less active. According to the “oxide” mechanism, a drop in the catalyst surface activity is attributable to the blocking of the “sites” for oxygen and carbon monoxide adsorption by the formed oxide. The reduction of the active sites takes place due to slow CO interaction with the inactive oxygen, which is part of the metal oxide. Thus, the fast oxidation and slow reduction of the catalyst surface causes transitions between the two steady states of the reaction rate generating the oscillations. It is also assumed that the oxide phase does not form under low pressures and the reaction transition to the oscillation mode is related to the formation of a “subsurface” form of oxygen.
Experiments using X-ray photoelectron spectroscopy conducted at the Institute of Catalysis SB RAS have revealed that the oxygen atoms do penetrate into the metal forming a special “subsurface” layer. Palladium oxide, however, does not form. The oxygen‑180 isotope and molecular-beam technique have helped the researchers to prove that the atomic form of the oxygen adsorbed on the surface is more reactive than the “subsurface” oxygen. The periodic formation and consumption of the latter gives rise to the critical phenomena: hysteresis, auto-oscillations and chemical waves
My team was mainly interested in energy conversion based on catalytic processes; at some point, an independent laboratory was set up to investigate the catalytic methods for solar energy conversion. It appeared with time that, apart from solar energy, nontraditional catalysis conceals other interesting things. This is why many specialists from my team detached themselves to form their own units but the laboratory has preserved its historical name.
Science as the basis for practice
Nearly all of the SB RAS research institutes were established not only for the purpose of pure theoretical studies but also in the best interests of the nation. The new research center, remote from the western border, was also viewed as a standby base of defense science. Virtually all the first research institutes (the Institutes of Hydrodynamics, Applied and Theoretical Mechanics, etc.) focused on defense; on top of that, there were chemical institutes serving the interest of atomic industry, geological institutes aimed at the development of Siberia’s mineral resources, and so on.
In April 1965, the Novosibirsk Chemical Plant started to make an experimental-industrial catalytic reactor for the production of formaldehyde (not from methanol) based of iron-molybdenum catalysts. The original design of the tubular reactor and new catalyst were developed by the researchers of the Institute of Catalysis in collaboration with the specialists of the plant. This was the beginning of a fruitful cooperation between the young research institute and industryOur Institute of Catalysis was formed a year later than the Siberian Branch by a special resolution of the Plenum of the Central Committee of the Communist Party of the Soviet Union, which promoted the development of chemical industry in the USSR. In line with it, the largest chemical plants of petroleum refineries were constructed and commissioned, 17 sectoral research institutes and 3 academic research institutes were launched. The Institute of Catalysis was tasked with the implementation of research results in chemical industry.
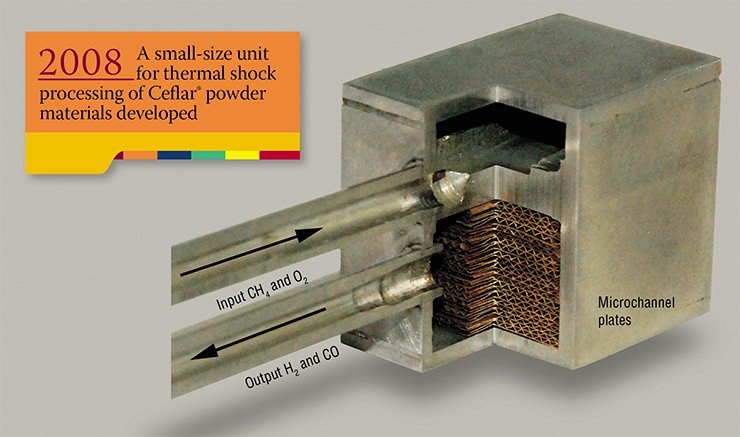
The science of catalysis is the most exciting area of chemistry since it is a mix of physical, organic and inorganic chemistry with material science and engineering. The two founders of the institute, G. K. Boreskov and M. G. Slinko, were not only outstanding chemists but also chemical engineers. From the very start, large production facilities were launched – pilot chemical departments, which lacked the Moscow institutes. Ten years later, the institute established a partner for the purpose of the industrial implementation of its research results – the Catalyst Special Design and Technology Bureau, which belongs to the Ministry of Chemical Industry.

There is every reason to believe that in the 21st century the fossil energy-carriers (coal, oil, and gas) will be substituted for hydrogen – a new environmentally sound fuel. The major obstacle to using hydrogen as an energy-carrier is that this gas in a free state is virtually unavailable in nature.
In order to switch to hydrogen energy, it is necessary, first of all, to develop efficient technologies for the large-scale hydrogen production, storage, transportation, and, second, to create new-generation power plants fueled with hydrogen. Another issue is the cost of hydrogen since its production so far is exceptionally material-intensive and energy-consuming. Therefore, it is critically important to find in the near future efficient ways to produce hydrogen and synthesis-gas, which contains hydrogen, from cheap and immediately available natural gas.
All highly efficient processes designed in the world for hydrogen and synthesis-gas production from natural gas inevitably involve catalysts. Even though focused research on the portable units for hydrogen production (“hydrogen processors”) started in Russia 10 to 15 years later than abroad, the national science is no doubt competitive in this area.
To illustrate, the Boreskov Institute of Catalysis, SB RAS, has developed highly efficient structured catalysts for the reaction of partial oxidation of methane. The catalysts are in the shape of strips or monoliths made of thermostable metal alloys and ceramics. Based on these catalysts, compact reactors for natural gas conversion have been developed, which allow a throughput of ca. 4 m3 of methane per hour per 1litre of the reactor volume.
Another promising development concerns the process of methane steam reforming. Since this reaction is endothermic and proceeds at high temperatures, it requires heat supply. To deal with this problem, a shifty design has been proposed: on the one side of a metal catalyst plate, the reaction of methane oxidation goes with heat release, and on the other side, the steam reforming of methane. The metal plate readily transfers the heat; as a result, the reactor productivity considerably increases. Based on this idea, the first fuel processor for high-temperature fuel cells has been developed in partnership with the Boreskov Institute of Catalysis, with financial support from Norilsk Nickel JSC MMC.
A promising fuel for portable fuel cells is sodium borohydride, from which hydrogen is generated in a catalytic reaction. The Institute of Catalysis has developed monolith and granulated catalysts for this reaction, which are on a par with the best foreign counterparts. In cooperation with the State Research Center of Chemistry and Engineering of Organoelemental Compounds (Moscow), pilot cartridges based on these catalysts have been made for feeding portable fuel cells.
Low-temperature fuel cells require exceptionally pure hydrogen, free of both CO and CO2. The idea proposed by the Siberian researchers is simple: to use an adsorbent that adsorbs CO and CO2 during the steam reforming of hydrocarbon fuel and obtain pure hydrogen at the outlet. If we use a pair of reactors-adsorbers, one of which operates in the adsorbing mode, and the other in the regeneration mode, the process will be continuous. The idea has been already put into practice.
Among other promising developments of the Institute are catalysts for methane pyrolysis with zero CO2 emissions, membrane reactors for natural gas oxidation by the oxygen that penetrates into a reactor from the air through a special membrane, and many others
After G. K. Boreskov’s unexpected departure from life in 1984, K. I. Zamaraev became the director of the institute and I became his deputy for science. In the mid‑1980s, the institute was awarded the status of the parent organization of the Catalyst Cross-Sectoral Research and Technology Center. This was something like a miniature ministry that supervised a subsector of the Soviet economy: 25 research institutes and plants engaged in catalyst production. I became deputy general director of the Center and had to get familiarized with applied problems – it was a steep learning curve.
In 1991 the former, “Soviet” life collapsed. Right before the New Year of 1992, Kirill Ilyich and I developed a strategy for saving the institute. Zamaraev supported my idea of focusing on the core of the institute, its promising researchers that would be able to raise funds (in any activity, as the phrase goes, it’s all about the people) rather than trying to save the institute as a whole, which was utterly impossible.

Today zeolites – natural and artificial minerals with a micro-porous crystalline structure featuring unique catalytic and adsorption properties – have a wide range of applications. A most promising way to make zeolite-containing materials for catalysis is growing zeolite crystals on a specially designed carrier with a network of transport pores; the size of the crystals to be synthesized should not exceed a few nanometers. As a result, virtually all active centers of the zeolites become “surface” with respect to the zeolite structure, and since in the course of synthesis the crystals attach to the ready-made carrier with large transport pores, their active centers become accessible for big molecules.
The technologies for the synthesis of nanocrystalline and “hierarchical” zeolites developed at the Institute of Catalysis SB RAS make it possible to create catalysts for the petrochemical industry. These catalysts allow for a selective removal of heavy hydrocarbons and increase in the number of light fractions, which are more in demand, in particular, for aircraft and diesel fuel production. The heavy residues can be decomposed into the lighter ones, which will yield more gasoline and oils. The associated gas, now burnt for nothing in immense amounts, can be catalytically “condensed” to benzene and other liquid “aromatic” compounds on oil-production sites.
We hit the mark: after the demise of the Soviet Union our research institute was virtually the only one within the Siberian Branch that continued its progressive development. Very illustrative was the year 1997. We got from the state only 17 % of the institute’s budget but paid more tax than in the previous years. Until 2000, while our economy was down in ruins, we collaborated mostly with Western partners: for quite some time, the Western Europe made polypropylene using our catalysts. When the Russian economy began to revive, our focus shifted to the national industry. Curiously enough, we managed to save the All-Union Research Institute of Technical Carbon in Omsk by integrating it with our Omsk Branch; today, this is the Institute of Hydrocarbon Processing Problems SB RAS. At that time, K. I. Zamaraev was away on a business trip, and by the time he came back, we had had an addition to the family.
 In the 1990s, I worked a lot with V. A. Koptyug including the period when I was director of the institute. In particular, the SB RAS rating and contract system was developed under his instruction.
In the 1990s, I worked a lot with V. A. Koptyug including the period when I was director of the institute. In particular, the SB RAS rating and contract system was developed under his instruction.
The greatest disadvantage of the latest R&D reform that took place in 2013 was the disruption of the coordination with other research institutes of the Siberian Branch. What is good about Akademgorodok is that all the institutes are literally within walking distance, and you can find specialists in nearly any research area. We used to have an efficient system of integrated projects, which now has to be restored.
Nonetheless, our institute has always been commercially viable, and because of it, we have a lot on our plate. Here are some of our achievements. Back in 2003 we were entrusted with one of the first mega projects and received from the state a vast amount of money – 500 million roubles. Our task was to develop modern domestic catalysts for oil processing. Using them, Russian industry produced, just within the three years of the project’s duration, over 8 billion roubles of high-octane gasolines. In other words, per each rouble invested in our project the state got 15 roubles added to its GDP! As far as I know, no other project in Russia has had a commeasurable investment efficiency.
The idea of making liquid fuels from renewable plant raw materials is not new. After the Second World War, however, a sharp increase in oil production resulted in cheaper costs of the gasoline and diesel fuel made using off-the-shelf oil-refining technologies. Yet, the switch to fossil hydrocarbons was not final or definitive. Today, about 80 % of the world’s ethyl alcohol is used as a fuel – a partial substitute for gasoline. Diesel fuel also has a substitute – biodiesel, made from methanol and vegetable oils through transesterification (exchange of fatty acids radicals) involving catalysts.
The most ecologically-friendly way to produce biodiesel requires hard, so-called heterogeneous, catalysts, which can be easily detached from the reaction products. In contrast to the homogenous catalysts, conventionally applied for biodiesel production, heterogeneous catalysts can be reused. Another plus is that biodiesel is of higher quality. Also, no preliminary processing of vegetable oil is required, which minimizes liquid waste. These catalysts, however, should meet specific requirements. The developers of heterogeneous transesterification catalysts from the Institute of Catalysis SB RAS put a special emphasis on their job stability in real-life conditions. Most promising in this respect appeared to be barium, calcium and lanthanum hexaaluminates, which are relatively low active but highly resistant to leaching.
Alternatives to transesterification in making biodiesel can be “soft catalytic cracking” (conversion of the feedstock hydrocarbons at high temperatures in the presence of catalysts) and “hydrocracking” (cracking in the presence of hydrogen). An advantage of hydrocracking is that it can be done in conventional petroleum refineries. In addition, since the composition and properties of the hydrocracking products of vegetable oils are similar to the hydrocarbons contained in standard diesel and gasoline fuels in combustion engines, they can be used together.
The hydrocracking of vegetable oils and fatty acids usually employs the commercial sulfided catalysts of oil refining. Because of the low content of sulfur in the plant feedstock, however, these catalysts are prone to a quick decay, and an intentional addition of sulfur compounds soils the target product. The Institute of Catalysis has developed a series of non-sulfide, nickel and copper catalysts that allow an efficient conversion of vegetable oils and their derivatives into hydrocarbon fuels at the same hydrogen temperatures and pressures as the commercial catalysts.
A typical product of wood waste processing is cellulosic ethanol. There is, however, a more efficient way of using the woodworking industry refuse as feedstock for fuel production if we depart from the usual choice between ethanol and gasoline. By means of rapid pyrolysis, a liquid product, conventionally referred to as “bio oil” (in Russia, this product has been known for ages as “tar”), can be produced from wood. Bio oil, however, is well-oxygenated and because of this cannot be used directly as motor fuel: oxygen should be removed from it and it should be saturated with hydrogen, i. e., hydrodeoxygenation should be performed. Within the framework of the international BIOCOUP project, specialists from the Institute of Catalysis SB RAS have proposed using for this purpose non-sulfided nickel-containing catalysts. The tests of nickel and copper-nickel catalysts developed by the Institute of Catalysis on model compounds and natural bio oil, conducted both in Russia and abroad, have clearly shown a distinct advantage of these catalysts in their basic parameters over the well-known commercial analogs.
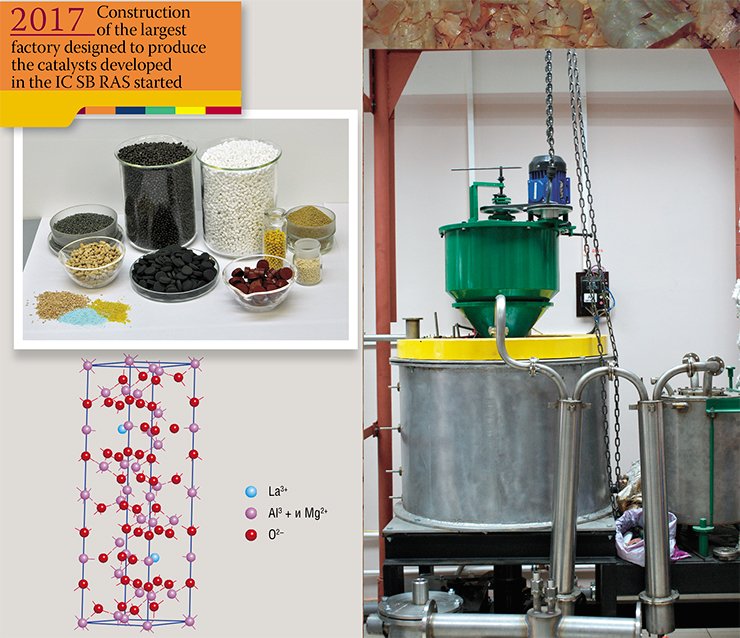
We have been the first in the country to make EHS (Extra High Strength) polyethylene. Our pilot production line has become the foundation for establishing an exclusive economic zone in Tomsk. Our technologies lie in the basis of five totally eco-friendly coal boiler houses that have been operating in Siberia for a few years. These may not be large projects yet but they are growing rapidly. And most importantly, the construction of the nation’s largest catalyst-making plant is starting at the premises of the Omsk-based Gaspromneft factory. All the catalysts to be produced have been developed by Siberian scientists from our institute and our spin-off, the Institute of Hydrocarbon Processing Problems.
It is a common complaint now that academic science does not produce good results and that scientists have degenerated. Life shows that this is not true: challenge brings strong people to the forefront.
 During the era of industrialization in the USSR, research went hand in hand with industry. The classics of Soviet R&D were brought to birth at the time of great challenges in geology and chemistry. When the country was getting ready for the war, specialists in defense appeared. After the war, when the task to develop atomic industry was set, there were people capable of dealing with it. Later came space exploration and development of oil and gas deposits in West Siberia.
During the era of industrialization in the USSR, research went hand in hand with industry. The classics of Soviet R&D were brought to birth at the time of great challenges in geology and chemistry. When the country was getting ready for the war, specialists in defense appeared. After the war, when the task to develop atomic industry was set, there were people capable of dealing with it. Later came space exploration and development of oil and gas deposits in West Siberia.
Clearly, national science featured a very tough artificial selection. It was a selection indeed, and it had a system. But the tasks were set by the state itself, which, as a rule, listened to professional advice. If the state does not set such tasks, big people will not be there.
The problem with today’s Russian R&D is that in the late 1980s the Academy of Sciences was left to its own devices; the state stopped deferring to the scientists’ opinion. Now, when the state has resumed its viable interest in various research fields, the scientists, who have been disoriented for the last twenty years, appear to be not quite ready for it.
The conflict between one’s own interests and the interests of the society derives from the logic of our “academic life.” As scientists, we want to do the things that interest us. For example, I give lectures at University and think that my greatest recent achievement is the textbook Thermodynamics of Non-Equilibrium Processes for Chemists. However, this is my private research interest. Interest in major projects must be fostered by the higher-ups.
The Academy of Sciences is now asked to identify priority areas. There is no need to ask us: after sanctions against Russia were introduced, we are heaped with orders from our industry. Sectoral research institutes collapsed, therefore, academic research institutes have to deal with the tasks they were not meant for. The right time for feedback has been lost.
Another major flaw in the current situation with national science is the lack of research coordination. In the Soviet years, research was governed in a well-orchestrated manner. There was a pool for accumulating “academic ideas” and tasks of the country – the State Committee for Science and Technology. It was placed above all ministries to inform the higher-ups. There is no matching organization now. Fortunately, the recently adopted R&D strategy outlines proper objectives, though does not specify the mechanisms for attaining them.
What is the way to solve any problem? There is an objective and specific tasks to be handled, and mechanisms to be tailored to cope with these tasks. Today’s system of national economy management looks very different.
A model for me is the legendary figure of Russian and Soviet chemical science, Academician V. N. Ipatiev (this year, we are celebrating his 150th anniversary). Lieutenant General in the tsar’s army during the First World War, he rapidly raised Russia’s military chemical industry to a high level and in this way supplied munitions to the country. After the October Revolution, he collaborated with the Soviets to restore the chemical industry of Soviet Russia, though he had never sympathized with the Bolsheviks. It was at that time, in 1921, that he made a statement in keeping with the current situation: “Production can be sustainable only if it relies on domestic raw material resources and national specialists.”

Ipatiev, who must have been an associate of Trotsky and who found himself in a political vacuum in the late 1920s, had to move to Germany and then to the USA, where he worked for the oil-processing industry. Eventually, Henry Ford admitted that Ipatiev had been the forefather of modern American civilization as he had created affordable technologies for the production of high-octane gasoline that underlie the development of automobile industry. These technologies gave the Alliance’s aviation an edge during the next world war: American planes were worse than the German but they had better gasoline.
It is a striking fact that Ipatiev has made four (!) attempts to go back to Russia but he failed. The outstanding scientist was stripped of his rank of an Academician, deprived of citizenship and excluded from the USSR for good. Despite this, he continued to love his country and believed that the most important thing for a true scientist is to work for the benefit of his or her nation. I think this is still true today.
References
Parmon V. N. Natural selection among molecules // SCIENCE First Hand. 2004. V. 0. N. 1. P. 32—41.
Parmon V. N. Thermodynamics of Non-Equilibrium Processes for Chemists. Moscow: Intellekt, 2015. 427 p.
Luzgin M. V., Rogov V. A., Parmon V. N. et al. Methane aromatization on Zn-modified zeolite in the presence of a co-reactant higher alkane: How does it occur? // Catalysis Today. 2009. V. 144. P. 265—272.






