How viruses can help cure cancer
In developed countries, oncological diseases are the second most common cause of mortality after cardiovascular diseases; however, they are likely to become the number one cause of death in the near future, as it has already happened in Japan. The reason is that the main causes of cardiovascular pathologies are well known, and the modern medicine has ways to tackle many of them. The situation with oncological diseases is more complicated: there are a number of genetic and ecological causes of cancer, and prevention in the most of the cases is still impossible; besides, there are no efficient methods of treatment for a number of tumors. At first, the idea of using viruses against tumors may seem absurd, because the very word “virus” conjures up illness. But take a closer look, and the idea looks promising, as long as we make sure that only cancer cells get attacked by viruses
The first reports about the use of viruses to treat cancer — including successful cases — were published in 1904—1910 (Dock, 1904; De Pace, 1912). Back then, scientists described accidental (and later, intentional) use of weakened (vaccine) strain of the rabies virus to treat cancer patients. However, this method never came to be widely used, because the viral strains used that time had significant side effects, and the outcome of such treatment was poorly predictable.
In the further course of the 20th century, there were several more attempts to employ this method. In the 1950’s through 1970’s, non-pathogenic viral strains of the West Nile fever, yellow fever, rabies, Newcastle disease viruses, adenoviruses, etc. were used in attempts to treat cancer. Some patients completely recovered or went into temporary remission. However, poor predictability and lack of scientifically based knowledge on the action of viruses on tumors, together with prejudice from skeptics and controlling government institutions, completely undermined these efforts.
The recent history of oncolytic viruses
The nineties saw a more conscious effort to create oncolytic viruses. It happened when scientists managed to partially discover the mechanisms of specific anti-cancer activity of ONYX‑015, the recombinant strain of adenovirus developed in the USA. It turned out that one of the chief defenders of the cell, the p53 protein, initiates apoptosis (programmed cell death) upon discovering the virus, so that the virus cannot replicate and contaminate the surrounding cells. In many tumors, the gene coding the p53 protein is damaged, and nothing halts viral replication. In its turn, the adenovirus has the E 1B‑55K protein, which binds to p53, preventing it from launching apoptosis. If the gene coding this E 1B‑55K protein is removed from the genome, the virus will only be able to actively reproduce in the tumor cells, which in many cases have a nonfunctional p53 protein.
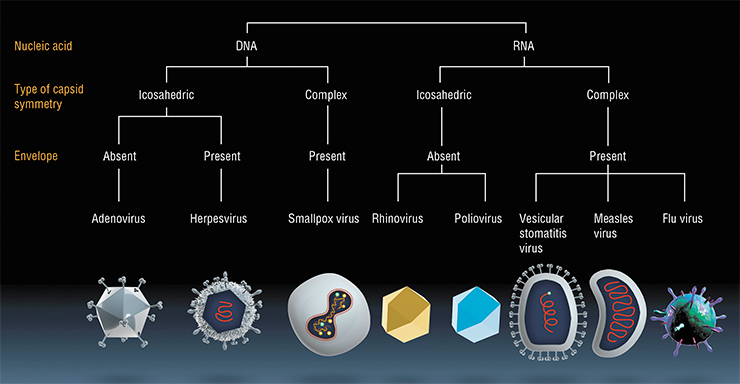
Eventually scientists discovered that with a lacking E 1B gene, the second function of the E 1B‑55K protein is not realized, either (O’Shea et al., 2004); that function is to transfer of viral mRNAs (which code proteins of the viral envelope) from the nucleus into the cytoplasm. In tumor cells, this function is overtaken by a yet undiscovered factor. This means that the mechanism of selective action of ONYX‑015 needs future investigation. Moreover, transition of healthy cells into cancer cells can be caused by reasons other than p53 protein gene defects. There are other mechanisms of cancerous transition, and in these cases, the E 1B-gene deleted adenoviruses will be ineffective.
 All this led to oncolytic virus research coming down to a halt by the late 1990’s. However, an analog of ONYX‑015 under the name of Oncorine was approved for the treatment of some types of cancer patients with head and neck tumors in China, as well as a recombinant adenovirus with a deleted E 1B gene and an inserted p53 gene to enhance its oncolytic properties (as the drug Gendicine) (Guo et al., 2006).
All this led to oncolytic virus research coming down to a halt by the late 1990’s. However, an analog of ONYX‑015 under the name of Oncorine was approved for the treatment of some types of cancer patients with head and neck tumors in China, as well as a recombinant adenovirus with a deleted E 1B gene and an inserted p53 gene to enhance its oncolytic properties (as the drug Gendicine) (Guo et al., 2006).
In the USSR, research of oncolytic properties of viruses began in the 1960—1970’s in the Institute of Poliomyelitis and Viral Encephalites of the Academy of Medical Sciences of the USSR (Moscow region). In addition to the study of polioviruses and developing polio vaccines, some vaccine strains of poliomyelitis virus were used to treat cancer. Moreover, a number of strains of enteroviruses with oncolytic properties and non-pathogenic to humans were isolated and characterized.
Marina Konstantinovna Voroshilova, a Corresponding Member of the USSR Academy of Medical Sciences, did a lot of work on oncolytic viruses; in several cases, she reached significant success, including complete elimination of the primary tumor and its metastases. However, in the 1970’s, her experiments were forbidden. The reason was the lack of data on the underlying mechanisms of the phenomenon and on the molecular nature of viruses and tumors interactions. Eventually, well after the end of her projects, she published two reviews, both in obscure journals (one Soviet and one foreign) (Voroshilova, 1987; Voroshilova, 1989).
VIRUSES: A “HEALTHY” COMPETITION “Until recently, it was a common belief that viruses cause diseases and should be treated as pathogens harmful for the society and requiring only ways of eliminating them. However, in the early 1950’s, this assumption encountered growing doubt; experiments on isolating viruses from feces of healthy children and studies of the properties of these viruses in primates, other animals and on tissue cultures produced amassing evidence that certain potentially pathogenic and completely non-pathogenic viruses (enteroviruses) can have certain properties beneficial for the host organism <…>
Our observations demonstrated that the majority of vaccinated* children showed good results, however, in some of them, the virus did not “latch” in the intestines, and antibodies did not appear in these children’s blood. Virological research conducted in our as well as in many other laboratories has shown that success of oral polio immunization by live Sabin vaccine was negatively impacted by competition from non-poliomyelitic types of enteroviruses present in the intestines of many children from birth and not affecting their health. This gave us a chance to confirm a symbiotic relationship between some races of non-pathogenic saprobic enteroviruses with the human organism; these viruses compete for the environment with the live Sabin vaccine and, in certain conditions, with pathogenic viruses <…>
We suggested a new principle of fighting enterovirus diseases by displacing pathogenic viruses with nonpathogenic enterovirus symbionts that would compete with the former for the environment; based on these benign strains, M. K. Voroshilova developed live enteroviral vaccines (Russian “живые энтеровирусные вакцины, ЖЭВ”) <…>
Our observations along with a number of studies proved the existence of a phenomenon new to science – the efficient suppression of pathogenic viruses with non pathogenic races of symbiotic intestinal enteroviruses due to their competition for the environment and interfering action along with stimulation of several defense mechanisms of the body. Beneficial viral symbionts developed through aimed selection have been found capable of causing oncolysis (destruction of some types of tumor cells) and in some cases of protecting the leukocyte system from the harmful influence of radiation. The discovery is important both for the general biological knowledge and for applied medical purposes, as it opens new possibilities for prevention and treatment of a number of diseases”.
Later, professor Viktor Vladimirovich Keshelava, while working in different Russian oncology clinics, used the Newcastle disease virus, which is non-pathogenic for humans, for treating some types of tumors; however, his experiments never went into clinical trials.
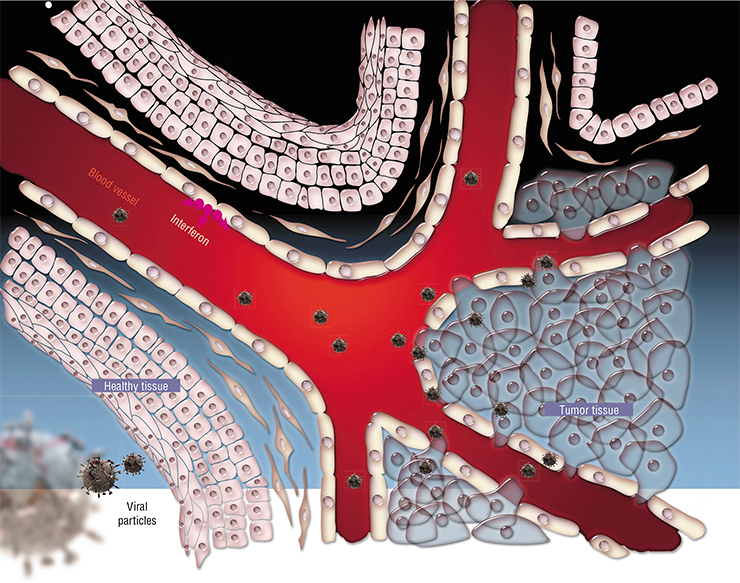
In 1998—2003, the State Research Center for Virology and Biotechnology “Vector” in Novosibirsk developed a variant of the serotype 5 adenovirus with a completely deleted E 1B gene. A preparation based on this virus was named Cancerolysin (Russian “канцеролизин”) (Kachko et al., 2003). It was shown to have oncolytic properties similar to those of ONYX‑015 and Chinese drug Oncorine. After full-size preclinical trials the strain was finally officially approved for phase I clinical trials (Vdovichenko et al., 2006), which took place in 2007 in the N. N. Blokhin Russian Oncological Research Center in Moscow on eight volunteer patients. The trials demonstrated good tolerance of the drug; in one case, there was therapeutic effect, even though all eight patients were at the incurable IV stage of the disease. However, searching for sources of funding for future trials failed, and several years later the research became irrelevant due to the emergence of a new generation of viral oncolytics.
Recent history: clinical trials
In the past decade, there has been a strong surge of research in the field of oncolytic viruses. One of the major breakthroughs was in October, 2015, when FDA approved a drug called IMLYGIC™ (Talimogene Laherparepvec, T-VEC), which is a type 1 Herpes simplex virus with attenuating mutations in its genome and insertion of the gene coding the human granulocyte-macrophage colony stimulating factor (GM—CSF) to enhance the anti-tumor effect. Information on the trials of this drug and its efficacy against other human tumors is regularly updated at www.imlygic.com. The drug is already available unofficially in Russia.
Another drug put into phase III clinical trials in 2015 was Pexa-Vec, JX‑594, which is based on a recombinant strain of vaccinia virus, intended for the treatment of hepatocellular carcinoma. Pexa-Vec is a recombinant derivative of the vaccinia virus with an added human GM—CSF gene and a removed gene for an the enzyme thymidine kinase to reduce side effects. Currently, Pexa-Vec is a FDA-designated orphan drug for Sorafenib-resistant hepatocellular carcinoma (Sorafenib, Nexavar; Bayer/Onyx), which is currently the only approved oncolytic virus-based drug for locoregional treatment of hepatocellular carcinoma. The orphan status of Pexa-Vec in the US and EU allows simplified market introduction procedures. Pexa-Vec is also being actively investigated on volunteers with other forms of cancer (information is available at http://www.sillagen.com).
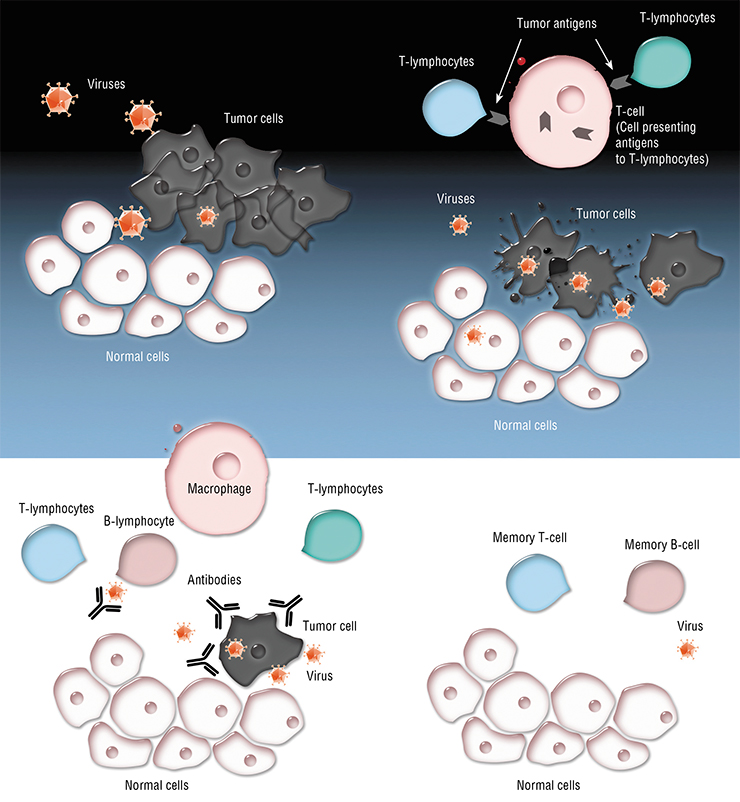
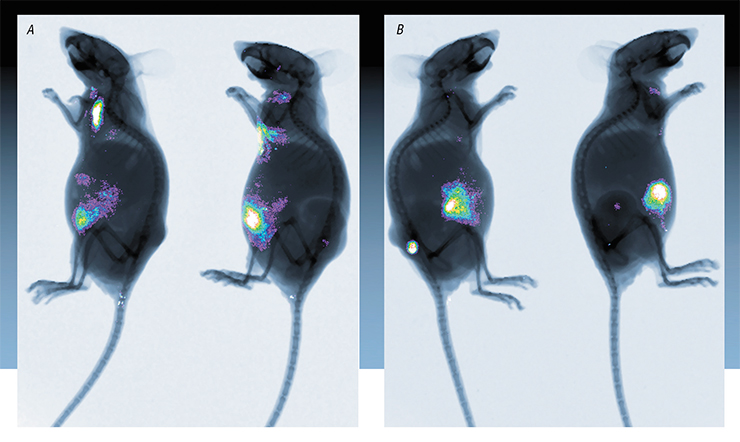
In Russia, scientists developed a candidate drug called VV-GMCSF-Lact based on the vaccinia virus (Kochneva et al., 2016) for treatment of breast cancer. This drug is a recombinant strain of the vaccinia virus in which two virulence genes have been deleted and replaced with the human GM—CSF gene and a gene coding the anti-tumor protein lactaptin. Lactaptin is a unique product of research conducted in the Institute of Chemical Biology and Fundamental Medicine of Siberian Branch of RAS (ICBFM SB RAS). It is a fragment of the human kappa-casein (Richter et al., 2015). In 2019, the VV-GMCSF-Lact strain successfully passed preclinical trials in Russia; in 2021, it will enter phase I of clinical trials as the first pharmaceutical drug for viral therapy of malignant breast tumors in humans.
Many countries are actively developing and running clinical trials of drugs based on other oncolytic viruses, such as the parvovirus (ParvOryx); reovirus (Reolysin); Coxsackie virus (Cavatak); enterovirus (Rigvir), etc.
In Russia, oncolytic viruses are studied and developed in Moscow (Engelhardt Institute of Molecular Biology) and in Novosibirsk (NSU, Vector, and ICBFM SB RAS).
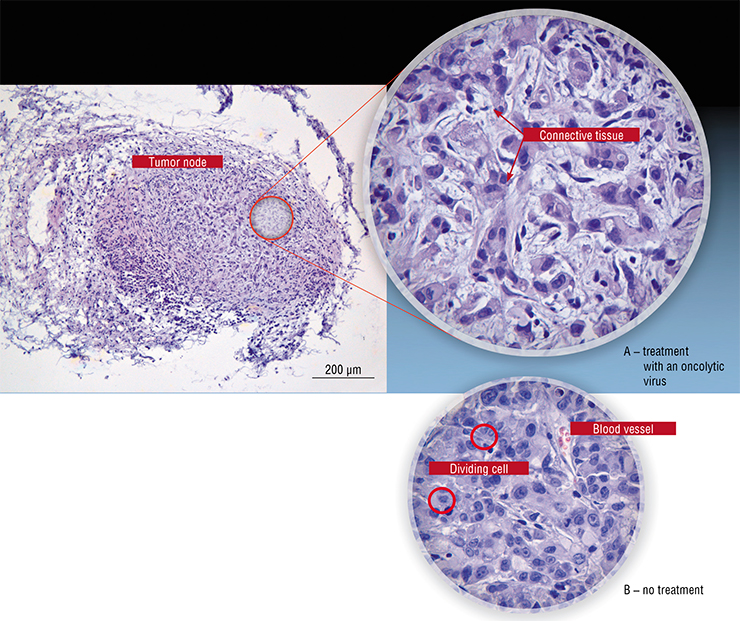
By the end of 2020, Russian scientists have developed a number of recombinant strains of the vaccinia virus with enhanced anti-cancer properties, which have shown potential in animal models (Kochneva et al., 2014; Kochneva et al., 2016; Kochneva et al., 2017; Tkacheva et al., 2019). A number of oncolytic strains of the Sendai paramyxovirus have been characterized and certified (Matveeva et al., 2015); a comparative study of anti-tumor properties of the serotype 5 and 6 adenoviruses was performed (Romanenko et al., 2019).
As it was pointed out above, a recombinant oncolytic viral strain based on vaccinia virus has passed all preclinical trials and has been approved for phase I clinical trials that are planned to begin in 2021. Today, Russian researchers have all the reasons to keep working: perform full-scale preclinical and clinical trials of oncolytic viral strains and continue engineering new variants of viral oncolytics. It is crucial to overcome all prejudice to greenlight future research and procure funding for the development of these extremely promising drugs developed in Russia.
However, there is strong distrust of the potentially useful cancer-battling viruses as fear of their pathogenic properties prevails. This is surprising, because many chemotherapy drugs widely used to treat oncologic diseases cause a wide range of deleterious side effects. The mechanism of action of most of chemotherapy drugs implies damaging not only cancer cells, but also actively dividing healthy cells. It is well known that chemotherapy often causes premature death of the patients; however, it is still used, as often there are no other ways to treat cancer patients.
This brings up another question of the role of viruses in our life. Some of them, just like bacteria, are present in our bodies, causing no harm. Others cause mild or even severe, but not very dangerous diseases destroying part of human organism cells. Could it be that the role of viruses, — or at least some of them, — actually consists in protecting us against cancer? What if they cause diseases only accidentally, by getting out of control? Answering these questions should become the direction of future research, if we really want to have a breakthrough in our battle with oncological diseases.
References
Dock G. Rabies virus vaccination in a patient with cervical carcinoma// Am. J. Med. Sci. 1904. V. 127. P. 563—565.
Guo J., Xin H. Chinese gene therapy. Splicing out the West? // Science. 2006. V. 314. N. 5803. P. 1232—1235.
Kochneva G., Zonov E., Grazhdantseva A. et al. Apoptin enhances the oncolytic properties of vaccinia virus and modifies mechanisms of tumor regression // Oncotarget. V. 5. N. 22. 2014. P. 11269—11280.
Kochneva G. V., Tkacheva A. V., Sivolobova G. F. et al. Antitumor potential of recombinant vaccinia virus strain, which produces a secreted chimera protein, composed of human GM-CSF and oncotoxic peptide lactaptin // Russian Journal of Biopharmaceuticals. 2017. V. 9. N. 1. P. 11—21.
Svyatchenko V. A., Tarasova M. V., Netesov S. V. Chuma-kov P. M. Oncolytic adenoviruses in anti-cancer therapy: current status and perspectives // Molecular Biology. 2012. V. 46. N. 4. P. 496–507.
Tarasova M. V., Demidova E. V., Kochneva G. V. et al. The construction of adenovirus type 6 vector with replication control under the human telomerase reverse transcriptase promoter. Collaborative Congress of the European-Society-of-Gene-and-Cell-Therapy (ESGCT) and Finnish-Society-of-Gene-Therapy. Helsinki, Finland. SEP 17-20, 2015. HUMAN GENE THERAPY. V. 26. N. 10. P. A101-A102.
Voroshilova M. K. Potential use of nonpathogenic enteroviruses for control of human disease // Prog. Med. Virol. 1989. V. 36. P. 191—202.
Vdovichenko G. V., Petrishchenko V. A., Sergeev A. A. et al. Preclinical studies of the anticancer adenovirus cancerolysin preparation // Vopr. Virusol. 2006. V. 51. N. 6. P. 39—42.