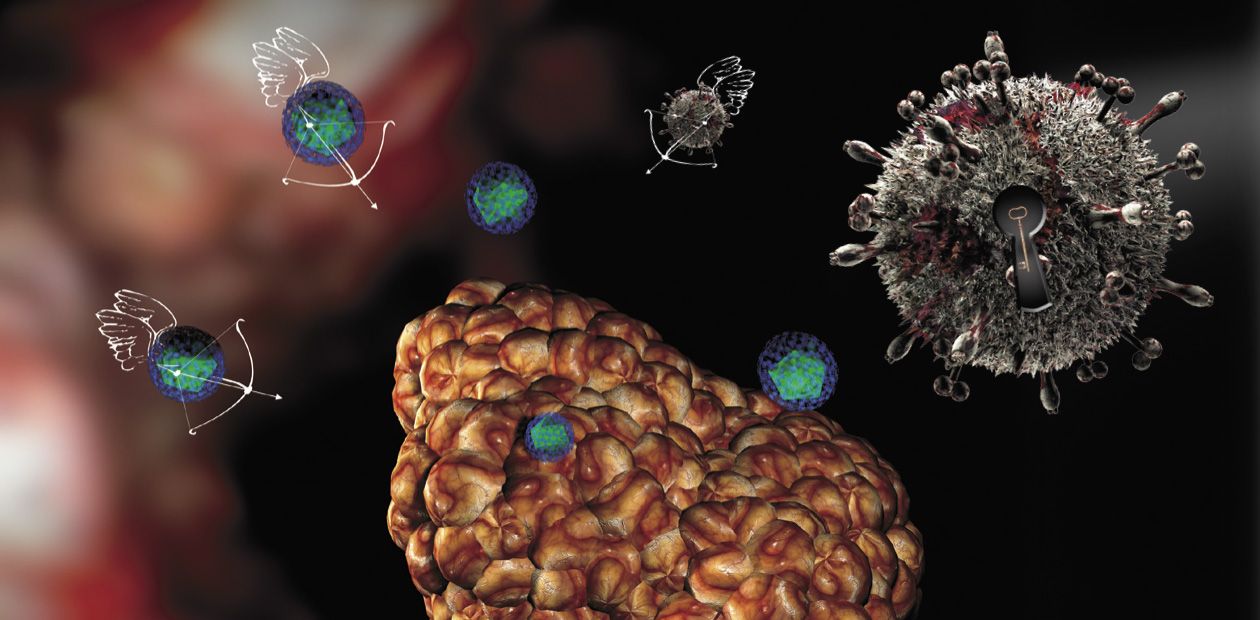
Viruses Attack... Tumors
The word “virus” usually brings to mind some specific and unpleasant associations, and this is not for nothing: the Latin equivalent of the word means “poison.” Dangerous, deleterious, pathogenic, and sometimes killing—that is how viruses (actually, just mobile sets of genetic information rather than full-fledged organisms) appear not only to the common people, but also to health professionals. This is not surprising: lacking their own systems for reproduction, the viruses necessarily parasitize cells of higher organisms. However, owing to this feature viruses have played an important role in the evolution of life and are nowadays essential in genetic engineering technologies. Yet in their usual role viruses can be our friends and healers, namely, when the cells they infect are malignant...
Our body is a sort of a microcosm, representing an environment for a multitude of finest creatures. However, while the bacterial community of the human body, comprising many friendly symbiotic or “neutral” species, has been actively and successfully studied, the concept of an analogous “viral community” is not generally accepted even among scientists
Nonetheless, numerous facts favor this statement. Look, for instance, at the widely known picornaviruses, which cause serious diseases, such as poliomyelitis and seasonal respiratory infections. It has emerged that these viruses, sometimes, are present in the intestine of healthy people too.
Moreover, a search for the infectious agent in the case of an outbreak of a viral disease often detects a real “bunch” of viruses rather than a single viral pathogen, so that occasionally it is very difficult to unambiguously state which particular virus is the cause of the disease.
Presumably, there are human virus parasites that reproduce in a limited manner in certain cell groups without doing much harm to their host. However, under certain conditions they are able to mutate forming more pathogenic variants. This brings about an acute viral infection, which comes to an end when the body forms a specific immunity to the corresponding pathogen.
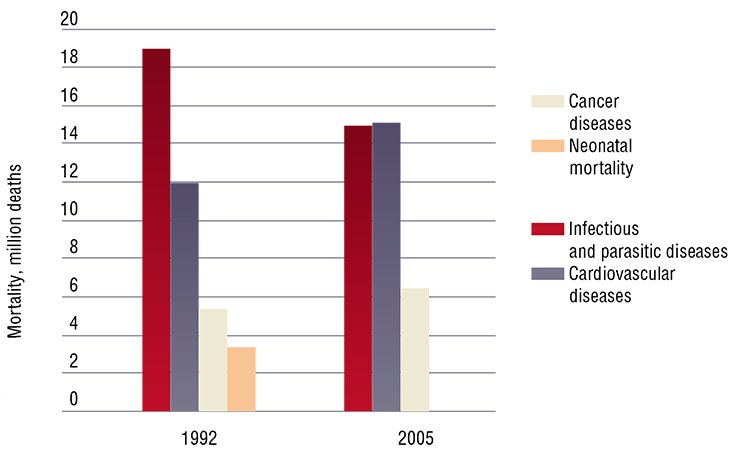
However, despite a relatively low level of knowledge about the virus community of the human body, an amazing fact was found quite soon after the discovery of viruses: a viral infection may influence the course of a malignant process in cancer patients. The cases of amelioration and even of a long-term remission have been recorded after a viral disease or a vaccination.
This information suggested, as early as in the beginning of the 20th century, the idea of utilizing viruses to control human cancer diseases.
Ups and downs
The feasibility of a fundamentally different kind of anticancer therapy, based on the characteristic ability of viruses to kill cells where they reproduce, was for the first time demonstrated in the mid-last century by the example of cervical cancer (Newman, 1954).

In Russia, the research into oncolytic properties of viruses was commenced in the 1960s—1970s at the Institute of Poliomyelitis and Viral Encephalitides with the USSR Academy of Medical Sciences (Moscow Oblast). Note that at that time a countrywide antipolio campaign involving various research laboratories was in full swing. In addition to studying poliomyelitis per se, they were involved in an extended research of the virus–virus and virus–cell interactions.
This made it possible to isolate and type several enteroviruses not pathogenic for humans, which were further used to design live enterovirus vaccines for the prevention of seasonal viral diseases. It is noteworthy that a positive clinical effect with respect to malignant diseases was observed in several cases, including a complete eradication of the primary tumor and metastases.
Further studies of vaccine therapy detected over a thousand and a half cases at late cancer stages with an amelioration in some of the patients (Voroshilova and Vaganova, 1969; Voroshilova et al., 1977; Voroshilova, 1988).
However, all the works on oncolytic therapy, both in this country and abroad, were ceased in the 1980s because of insufficient data on the mechanisms underlying this phenomenon as well as due to the technological difficulties in the purification of viral preparations required to guarantee patients safety. Figuratively speaking, they were afraid of “letting the genie out of the bottle.”
However, as early as in the beginning of the 1990s, oncolytic viruses again became an object of careful attention of researchers; this occurred after the paper on curing glioblastoma by modified herpes simplex virus (Martuza, 1991) was published.
VIRUSES: A “HEALTHY” COMPETITIONOur observations have shown good results for the majority of vaccinated* children; however, the vaccine virus was not “accepted” in the intestine of some children and no antibodies appeared in their blood. Virological studies performed in both our and many other laboratories demonstrated that competition with non-polio types of enteroviruses living in the intestines of many children from the moment of their birth without any harm to their health have and without any adverse effect on the success of oral immunization against poliomyelitis with Sabin’s live vaccine. This fact allowed us to confirm symbiotic relationships between the human organism and some races of nonpathogenic saprophytic enteroviruses, able to compete with Sabin’s live vaccine as well as with pathogenic viruses under certain conditions…
We have proposed a new principle for controlling enterovirus diseases by displacing pathogenic viruses with nonpathogenic enterovirus symbionts competing in their habitat. Using these symbionts, M.K. Voroshilova has created the so-called live enterovirus vaccines (LEVs)…
Our observations and several studies have for the first time confirmed the existence of the phenomenon novel for science, namely, an efficient inhibition of pathogenic viruses by nonpathogenic intestinal enterovirus symbionts as a result of competition for habitats and interference effect with concurrent stimulation of several protective systems of the body. Selected beneficial symbiotic viruses have emerged able to induce oncolysis (destruction of certain tumor cell types) and, in addition, to protect in some cases the leukocytic system of the body from a harmful impact of ionizing radiation. This discovery is of both theoretical biological and practical medical value, providing for new prospects in the prevention and therapy of several diseases.”
Over the following decades, a considerable advance in molecular biology and genetics and a rapid progress in research biotechnological methods made it possible to do tremendous experimental work which gave an insight into specific interactions between the oncolytic viruses and cancer cells and clarified the virus strains promising for anticancer therapy and the tumor types they are able to affect.
An “unprotected” tumor
Why on earth viruses (and not just some specific group of viruses but all of them) do first and foremost attack the tumor tissue? There are several reasons for this.
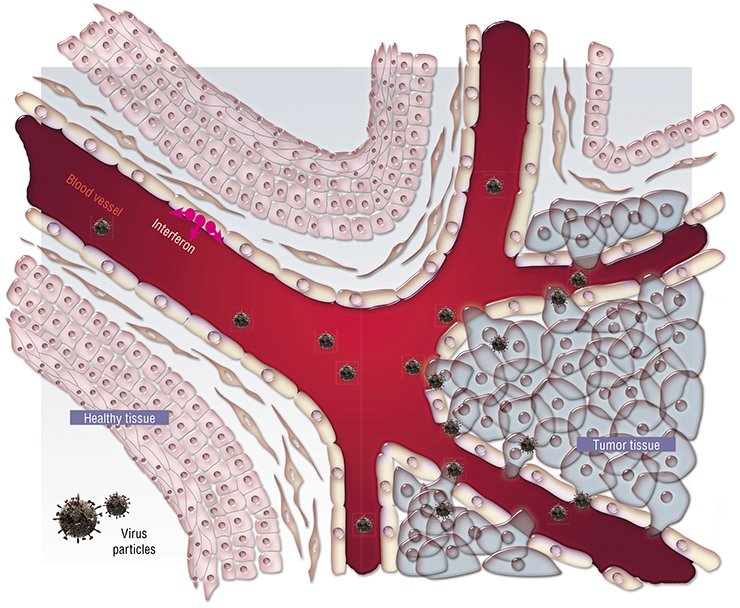
First, this is an unusual cell “pattern” of the tumor itself. All normal tissues and organs typically display a distinct structure and spatial arrangement owing to the presence of manifold membranes, cell arrays, blood vessels, and so on. All these structures represent natural barriers for spreading viruses. As for a tumor, its structure is, on the contrary, disarranged, representing a chaotic cell aggregation supplied with blood via rapidly growing flawed vessels with perforated walls. As a result, it is easier for a virus to enter a tumor and to spread there than to enter a healthy tissue.
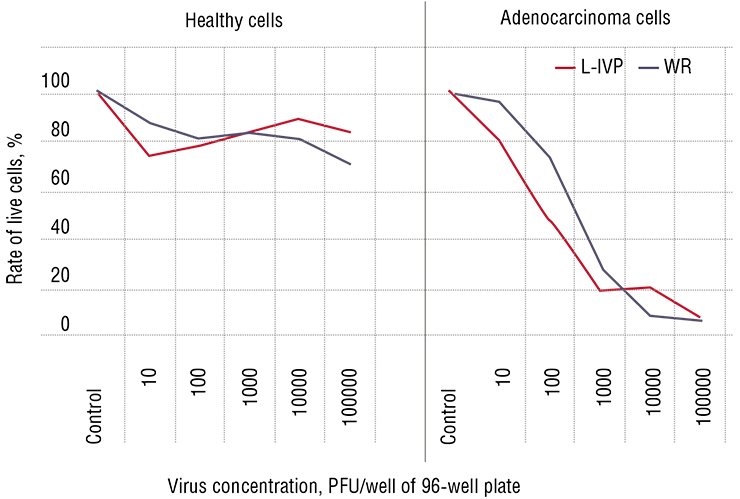
Thus, the first reason for a high sensitivity of cancer cells to viruses is their physical accessibility.
The second reason is associated with the types of reproduction of the infectious agents themselves. The case is that viruses are not self-sufficient in this respect and they utilize the machinery of an infected cell. Usually, this coincides with cell division, when the cell contains all the enzymes required for the replication of genetic material and protein synthesis.
Some viruses have specialized mechanisms allowing a cell to be switched from a dormant state to division (including the so-called oncoviruses, for example, papillomavirus, which causes cervical cancer via stimulating cell division). However, most viruses are able to reproduce only in actively dividing cells, for example, mucosal cells (which, as is known, are the first to be affected in seasonal infections). In this sense, the cancer cells, whose main duty is to divide, are especially unprotected from virus intervention.
Third, one of the most important distinctions of a normal cell from a cancer one is in that it “feels” like part of a whole. That is why some of its functions, though a burden on the cell itself, are necessary for the functioning of the overall organism. Among such functions is antiviral defense. As for the cancer cells, they often get rid of everything unnecessary during their development: their overall activity is exclusively aimed at unrestrained division and expansion. Since antiviral defense imposes numerous restrictions on the cell, the majority of cancer cells, as a rule, have impaired antiviral resistance.
The healthy are safe
Thus, by the grace of Mother Nature cancer cells serve as a ready-made target for viruses. Using state-of-the-art biotechnologies, it is now possible to design the viral “drugs” that would specifically affect and kill only cancer cells, skipping the healthy ones.
So, how is it possible to obtain an oncolytic drug safe for the normal tissues? It is known that some cancer cells start to express on their surface the proteins untypical of the normal cells, and it is possible “to teach” a virus to recognize such proteins. However, first, this property of cancer cells is not universal and, second, cancer cells are capable of “evolving” rapidly.
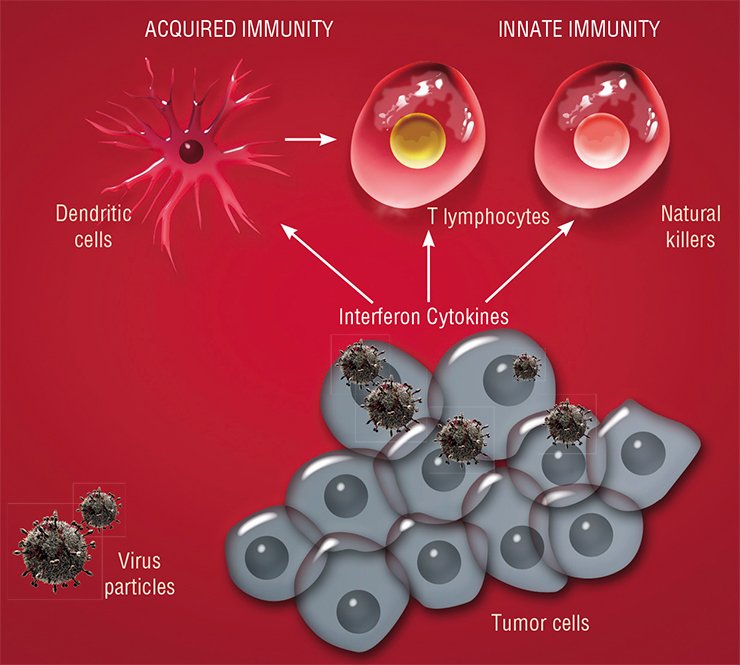
Researchers are ever more convinced that there are no two identical tumors in nature even if they are histologically most similar: cancer is induced by a damage of hundreds of various genes in a cell, variously arranged and having various variants. Moreover, even cancer cells of the same patient may display certain genetic and, correspondingly, phenotypic diversities.
Therefore, it is senseless to arm an oncolytic virus with the same “weapon”: the larger the number of targets that it will recognize on the cell surface, the wider will be its range of action. The universal characteristics of cancer cells associated with their malignant transformation should form the background for oncolytic virus selectivity. As for the major distinguishing characteristics of cancer cells, they are, as has already been mentioned, unrestrained division and undifferentiated character of tumors.
Thus, chaotic aggregations of actively dividing cells should be targets for viruses. There, the least differentiated and most malignant cells will be hit first, whereas the healthy differentiated cells will be protected from the attack of such a defect virus by antiviral defense mechanisms.
Custom-tailored service
In addition to negative selectivity for healthy cells, an “ideal” oncolytic virus should also display a wide range of action towards different cancer cell types. This can be achieved in different ways. The work strategy of Moscow and Novosibirsk teams consists in the construction of a panel of oncolytic enteroviruses with different antigenic structures covering a maximally wide range of cancer types.
In particular, to solve this problem, viruses are selected for their ability to reproduce in different cancer cell cultures. An analysis of genetic structure of such viruses allows us to assess which particular mutations acquired by a virus make it most efficient towards a particular cancer cell type.
Once a panel of such oncolytic viruses is available, it is possible to select the most efficient set of viral strains for this individual case by sampling the tumor of a cancer patient. Note that this procedure can be repeated during the therapy, since the tumor itself can change while escaping the oncovirus. In this case, another virus variant may be helpful, and since it will differ from the first one in its antigenic structure, it will escape the antiviral immunity mechanisms formed during the previous treatment.
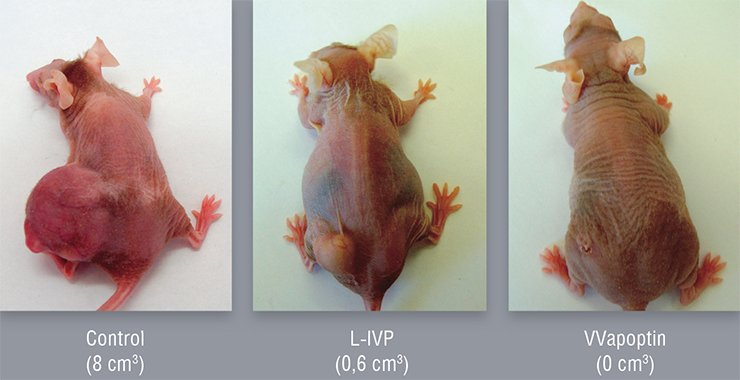
Currently, Cancerolysin undergoes phase II clinical trials. However, despite the evident efficiency of this and the like drugs designed in the late 20th century, they may be regarded only as a touchstone in the field of oncolytic viruses. Armed with state-of-the-art molecular genetic knowledge, today’s scientists can much better envision the strategy and tactics for creating such virus drugs
Another approach to the creation of oncolytic viruses may be referred to as “intracellular chemotherapy”. The commonly used chemotherapy is highly toxic, first and foremost, affecting the actively dividing cells of hematopoietic organs and mucosa. However, a specialized gene can be inserted into this virus selectively attacking only tumor cells; this gene will provide for an accumulation of a safe (until the time comes!) substance in the tumor cell. Then, interaction with another safe substance, which may be administered to the patient in a systemic manner and at high doses, in the tumor cell infected with the virus will give a highly toxic compound. Thus, a pinpoint therapeutic effect directed exclusively towards tumor cells can be attained.
Oncolytic viruses can be additionally armed with the genes producing the proteins in the cancer cell that are dangerous for these cells only. For example, the protein apoptin, isolated from the chicken anemia virus, induces suicide of cancer cells exclusively via a yet vague mechanism. Such a strain capable of killing efficiently human tumor cells grafted to nude laboratory mice has been genetically engineered using the vaccinia virus.
Another challenge in oncolytic virus therapy is associated with the elaboration of the most efficient methods for their delivery, since the virus has many barriers to overcome on its way inside the body. For instance, the very liver is a kind of most powerful antiviral filter.
A “Trojan horse” strategy may be helpful here, when the patent’s cells (for example, leukocytes) infected beforehand with the virus are applied rather than a pure virus. Having entered the body, the virus that just started to reproduce in leukocytes is “invisible” to the defender cells, and when the infected cell reaches the tumor, it releases thousands of active viral particles which attack the cancer cells.
Pro and contra
Currently, the use of oncolytic viruses in cancer therapy is an exception rather than a rule despite the progress in studying these viruses.
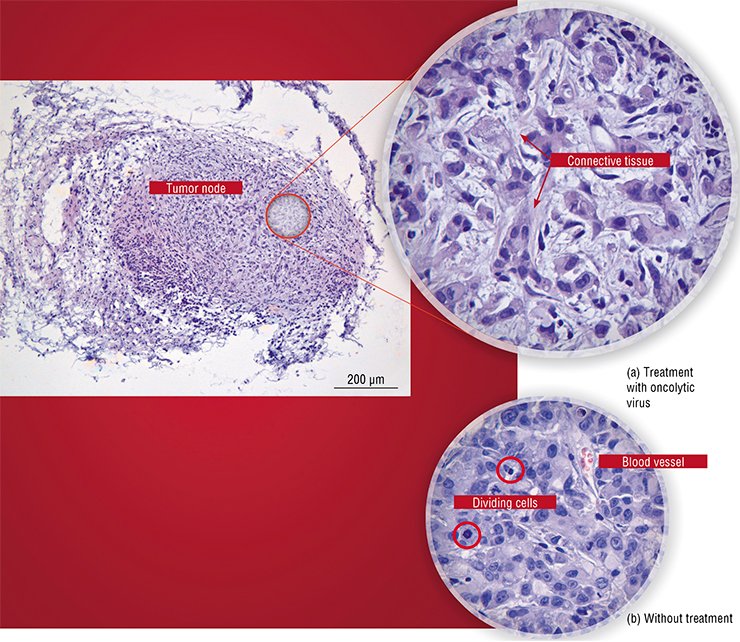
In particular, a preparation designed in the United States is now administered subject to the consent of the patient as an additional therapy when treating glioblastoma. An adenovirus strain carrying two genetic deletions was licensed and is successfully used in carcinoma therapy in China (Yu, 2007).
Research into oncolytic viruses, commenced within antipoliomyelitis activities, was continued in Latvia after the demise of the Soviet Union. As a result, an enterovirus-based preparation named Rigvir has been designed and is used in medical practice; Rigvir is intended for curing melanomas and can be injected directly into the blood stream.
Totally, over 30 different oncolytic virus-based drugs are now undergoing clinical trials worldwide. The main problem here is of economic rather than of scientific nature. The fact is that any cancer therapy (first and foremost, chemotherapy) is an issue of big money. Under the conditions of the established and prosperous chemotherapy market, the use of any new even safer biotherapeutics will require rather expensive rebuilding of the overall treatment system. And this cannot but affect the interests of major pharmaceutical companies.
The strategy of such companies towards the nascent developments associated with oncolytic viruses is ambiguous. In particular, the rights for one of the first oncolytic drugs, ONYX-015, were sold to a known pharmaceutical company together with the developer company, and further research was abandoned. On the other hand, since the progress in this field cannot be completely arrested, many pharmaceutical companies organize departments for studying viral oncolytics. The fact that the number of works in this field during last 15 years is snowballing also adds optimism.
Concurrently with the progress in engineering anticancer virus-based drugs, various phobias and rumors emerge as in the case of the notorious genetically modified organisms (GMOs). In many respects, they stem from insufficient information about the essence of this issue as well as are exaggerated by the efforts of some interested companies.
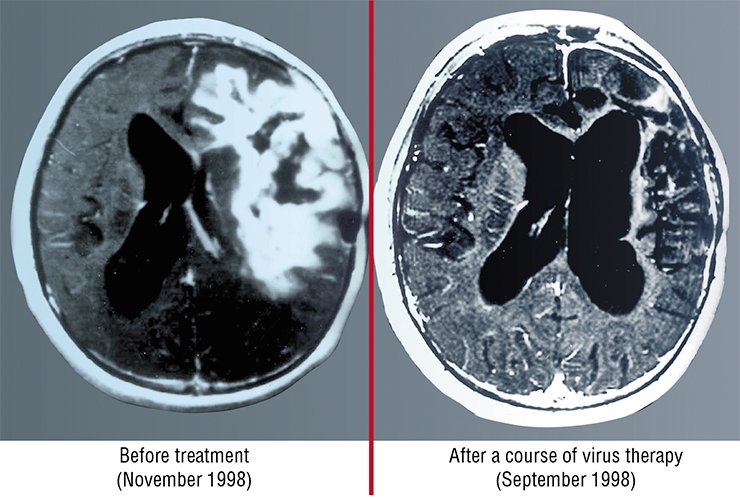
Indeed, the issue concerning the safety of particular viruses is to be further examined. Therefore, they have to accept that many barriers are to be overcome on the way to a wide application of these most promising cancer therapies. Anyway, researchers have to act in a most thoughtful manner, carefully weighing all possible consequences.
It is most likely that even when this cancer therapy approach is regarded as deserving wide application, certain epidemiological safety requirements should be met similar to those existing in infections hospitals, namely, strict virological control and limited contacts with patients. In addition, it is reasonable to construct such variants of oncolytic viruses that can be immediately “switched off” when it is necessary to prevent their chronic carriage.
However, according to the history of science, any new promising therapy has encountered such problems and, as a rule, succeeded in overcoming them.
Confidence is now growing that the knowledge accumulating in the fields of virology, immunology, cell biology, and other allied subjects will make it possible (and already does!) to engineer the virus variants that will not only efficiently and selectively kill cancer cells skipping the healthy ones, but also stimulate the natural anticancer defense mechanisms of the body.
In the future, artificial viruses may form the basis for cancer therapy. Today, the construction of a module-based viral system (in the future, completely synthetic) – a kind of a molecular meccano, using which a researcher can construct the desired oncolytic virus variant of individual genetic components – is a long-term target for the research teams from Moscow and Novosibirsk.
Such synthetic viruses should not structurally resemble any natural ones. This is important, since similar viruses are able to recombine with one another, exchanging parts of their genomes, conveying deleterious genes and, correspondingly, undesirable dangerous features to therapeutic viruses. It is possible to design a synthetic virus with its functions and structural plan similar to a natural one but drastically differing in its nucleotides sequence to make unfeasible any recombination with its natural fellows.
All this is a matter of rather remote future. As for today, viral oncolytics should not be regarded as a panacea. They can be an efficient supplement to the integrated attack on tumors in combination with the traditional therapies. Surely, such a viral “weapon” will be anything but needless in the control of a most deadly and hardly curable disease of our time.
References
Vdovichenko G. V. i dr. Doklinicheskie issledovanija protivorakovogo lechebnogo adenovirusnogo preparata «kancerolizin» // Voprosy virusologii. 2006. № 6. S. 39—42.
Voroshilova M. K. Poleznye dlja organizma nepatogennye shtammy jenterovirusov: profilakticheskoe i lechebnoe ih primenenie. M.,1988. S. 24—29.
Kachko A. V. i dr. Rekombinantnaja plazmidnaja DNK pAd5 f, nesushhaja fragment genoma adenovirusa 5 tipa s deleciej v gene E 1 V 55 K, i shtamm mutantnogo adenovirusa Adel2, obladajushhij selektivnoj protivoopuholevoj aktivnost’ju. Patent RF. № 2194755 (467) 05.03.01
Kachko A. V. i dr. Varianty adenovirusa tipa 5 s delenijami v rannih genah: sposobnost’ k selektivnoj replikacii v r53 defektnyh opuholevyh kletkah cheloveka // Molekuljarnaja biologija. 2003. № 37 (5). S. 868—875.
Martuza R. L. et al. Experimental therapy of human glioma by means of a genetically engineered virus mutant // Science. 1991. V. 252. P. 854—856.
* Sabin’s vaccine was used for immunization against poliomyelitis