Human Eye Lens: A Solar “Eclipse”
Photophysical and Photochemical Processes in the Lens of Eye
Sunlight plays a crucial role in the life of all organisms on our planet that would not be possible without photosynthesis enabling the production of organic compounds from basic elements. The heating of Earth’s atmosphere is another important contribution of the Sun to our lives. Finally, the sun’s rays lift our mood on a bright day. But sunlight may also be harmful. Solar radiation, primarily its ultraviolet component, can also affect the human body in a detrimental way, causing sunburns and even skin cancer. Furthermore, our organs of vision, which can not be protected by clothing like skin, inevitably experience regular and prolonged exposure to solar UV radiation. Do our eyes have a natural defence against the insidious light? If so, how does this defence work and how does its efficiency change as we age?
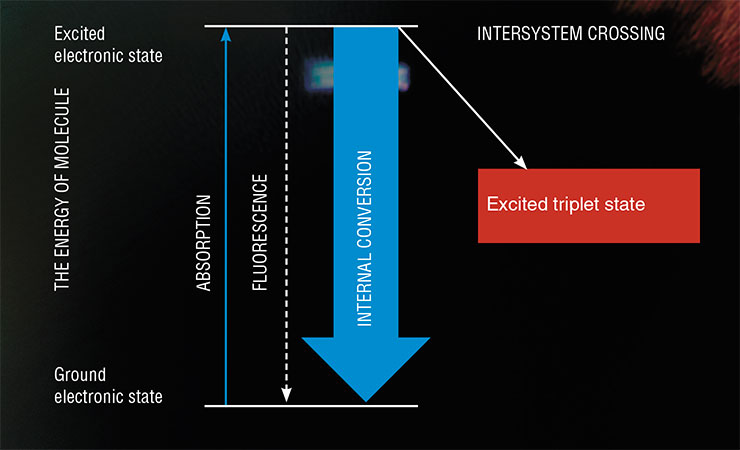
Why is ultraviolet radiation dangerous to humans? Due to the absorption of light by an organic molecule (e. g., a protein), one of its electrons (i. e., negatively charged elementary particles that populate atomic orbitals with different energies) moves to a higher energy level. This process is called the transition from the ground state to the excited state. This transition is benign to the human body, however the threat comes from the path that the molecule takes to return from this extremely unstable state to the ground one.
A light-excited molecule finds itself at a crossroads of three paths. First, the molecule can return to its original state through fluorescence, i. e., the emission of energy in the form of a quantum of light. The second option is to spend the excess energy on heating the environment by undergoing the so-called internal conversion. Both processes are quite safe for a living body since they do not initiate any chemical reactions. But the third path…
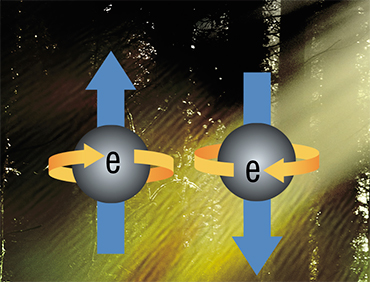
Before talking about it, let us recall the quantum concept of spin, which means the angular momentum inherent in elementary particles. The spin is, however, not associated with the real motion (rotation) of particles in space and therefore has no analogy in classical mechanics. Due to its quantum nature, the spin can only take on discrete, strictly defined values. For example, the spin of an electron is 1/2.
An electron's spin is associated with the multiplicity phenomenon, which is calculated for a molecule as the total spin of all its electrons plus unity. Under normal conditions, all the electrons in most molecules have a pair and, accordingly, a zero total spin. Thus, the multiplicity of molecules is 1, and this is the so-called singlet state (S-state).
However, when one of the electrons transitions to a higher energy level (i. e., the entire molecule finds itself in an excited state), two electrons in the molecule no longer have a pair. In this case, the multiplicity of the molecule depends on the projection of the spins and can be either 1 or 3. A change in the multiplicity from 1 to 3 indicates the transition of the molecule from the singlet to the triplet state (T-state), and the process of changing the spin state in a molecule is called the intersystem crossing.
This process represents the above-mentioned third path, along which unwanted reactions may be triggered. According to the principles of quantum mechanics, the transition from an excited T-state to the ground S-state is forbidden, which allows a T-state to exist over a long time. Such a molecule with an excess of energy becomes a hazardous particle capable of reacting with many of its neighbours. By the way, a molecule in the S-state is also capable of initiating chemical reactions, but the lifetime of such a state is so short that the molecule hardly ever has enough time to meet a reaction partner.
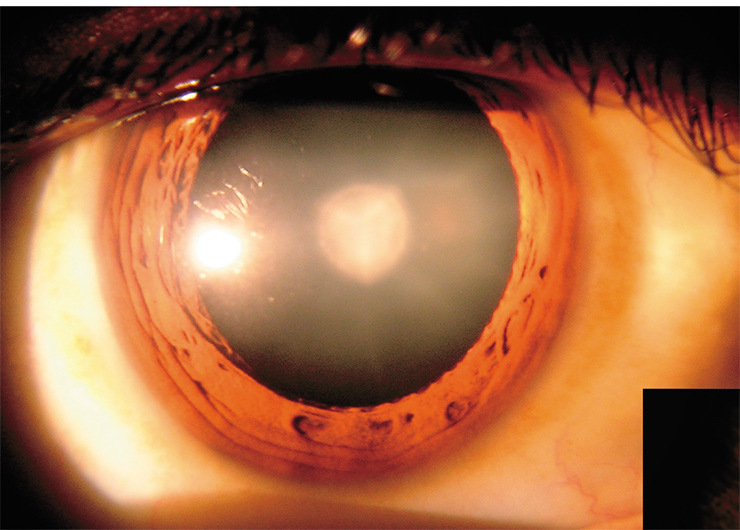
Chemical reactions from T-state may be very diverse and depend primarily on the molecule’s neighbours. As a rule, these reactions are irreversible, causing damage to molecules, primarily proteins and lipids since they occur most frequently in living cells. The accumulation of defective molecules may initiate, over time, the development of various pathologies, such as skin cancer and cataracts, i. e., a clouding of the eye’s lens.
Light into heat
The eye of mammals and humans is a complex organ consisting of several intricately organized parts. The main optical element is the lens, or crystalline lens, which transmits and focuses light on the retina surface. But this function is not the only one.
In the 1970s, it was found that the lens operates as a light filter by absorbing radiation in the near ultraviolet range (UV-A radiation, 315–400 nm). This radiation makes up about 95 % of all UV radiation on the Earth’s surface and, opposite to a shorter wavelength radiation, it is not absorbed by clouds and window glass. Moreover, this part of UV radiation is able to initiate photochemical reactions that cause damage primarily to photosensitive retinal cells.
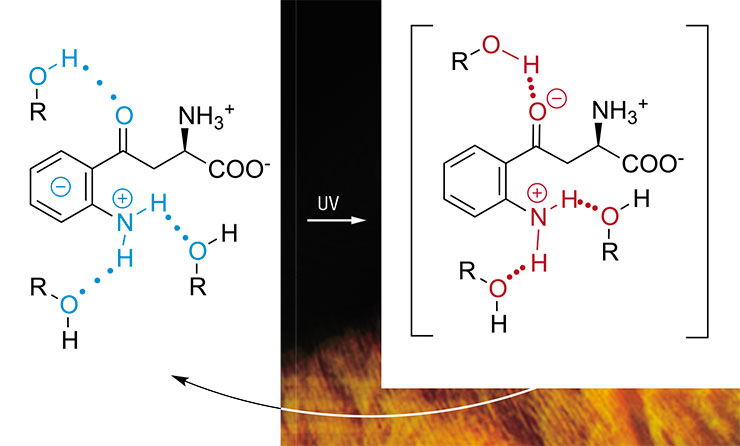
This discovery became possible through the effort of the Dutch researcher Ruth van Heyningen, who first showed that the human lens contains large amounts of kynurenine (an intermediate product of the enzymatic breakdown of the tryptophan amino acid) and its derivatives. These pioneering studies showed that kynurenines absorb radiation in the UV-A range without any detectable emission i. e., kynurenines serve as molecular UV filters. However, the mechanism of how they work remained unknown until recently.
The photophysical and photochemical studies at the International Tomographic Center, Siberian Branch, Russian Academy of Sciences (Novosibirsk) have shown that in aqueous solutions under the action of UV-A radiation, kynurenine glows weakly (Sherin et al., 2009) and produces an extremely small amount of reactive triplet states (Tsentalovich, 2008). This means that kynurenine returns from an excited state to the ground state mainly by converting light energy into heat (i. e., by means of internal conversion), and it does so very quickly and efficiently.
To understand how this happens, kynurenine’s behaviour was studied in a wide range of solvents. We found that the rate of transition of this molecule from an excited state to the ground state directly depends on the ability of the solvent to form intermolecular hydrogen bonds in which the hydrogen atom links together two electronegative atoms. Thus, the transition time in an aqueous solution was 30 ps (1 picosecond = 10–12 seconds) while in alcohol solutions, it was an order of magnitude longer. In solvents that cannot be a donor of intermolecular hydrogen bonds, the transition time increased by a factor of 100! In other words, intermolecular hydrogen bonds ensure the dominance of internal conversion in the photophysics of kynurenine.
Understanding how kynurenine works as a molecular UV filter was important from the perspective of photobiology. It became clear that this compound needs a medium saturated with intermolecular hydrogen bonds, such as alcohol or water. In the absence of hydrogen bonds, molecules in the excited singlet state pass into reactive triplet states (Sherin, 2009). When it happens, the light filter turns into a photosensitizer. When exposed to light, a photosensitizer can damage molecules from its immediate environment, which are mainly proteins in the case of an eye lens.
Ageing of the eye’s lens
Studies by Australian scientists showed that the abundance of kynurenines in the human lens decreases substantially with age (Bova et al., 2001), largely due to their attachment to proteins (Korlimbinis et al., 2007). This is a natural process resulting in the accumulation of kynurenine-modified proteins in the lens. Speaking about this “attached” kynurenine, one might ask: What is it in fact – an effective UV filter or a photosensitizer?
Experiments using kynurenine with artificially attached amino acids or proteins showed that the time during which kynurenine’s excited state “dies” depends on the bulk volume of the attached molecule (Sherin et al., 2010). The larger the volume of the substituent molecule, the less opportunity remains for kynurenine to establish intermolecular hydrogen bonds. And this, in turn, slows down the process of internal conversion of the absorbed light energy into heat.
In the case of a protein, the most important is the location of kynurenine inside a large and folded macromolecule – the deeper the location of kynurenine, the longer the lifetime of its excited state and, consequently, the greater the chances for it to pass into a reactive T-state. Thus, deeper penetration of kynurenine inside the lens proteins enhances its photosensitizing properties.

The formation of the triplet states of kynurenine is the first step towards the modification of proteins inside the lens cells, which are unique in the human body. The lens cells are, in fact, transparent fibres up to 1 cm in length that stretch from the anterior to posterior surfaces of the lens, tightly adjacent to one another like bulbous scales. A distinctive feature of these cells is the absence of cell nuclei or organelles that could scatter light and reduce light transmission. Thus, the lens cells have no biochemical machinery for the synthesis, restoration, and utilization of proteins; therefore, the lens proteins, or crystallins, are not renewed throughout human life.
With age, our eye lens proteins accumulate numerous chemical modifications, leading to substantial changes in the lens properties, such as increased rigidity, yellow colouring, and increased light scattering. However, until recently, it remained unclear what changes in the proteins’ structure were due to light-excited kynurenine molecules.
How light “stitches” proteins together
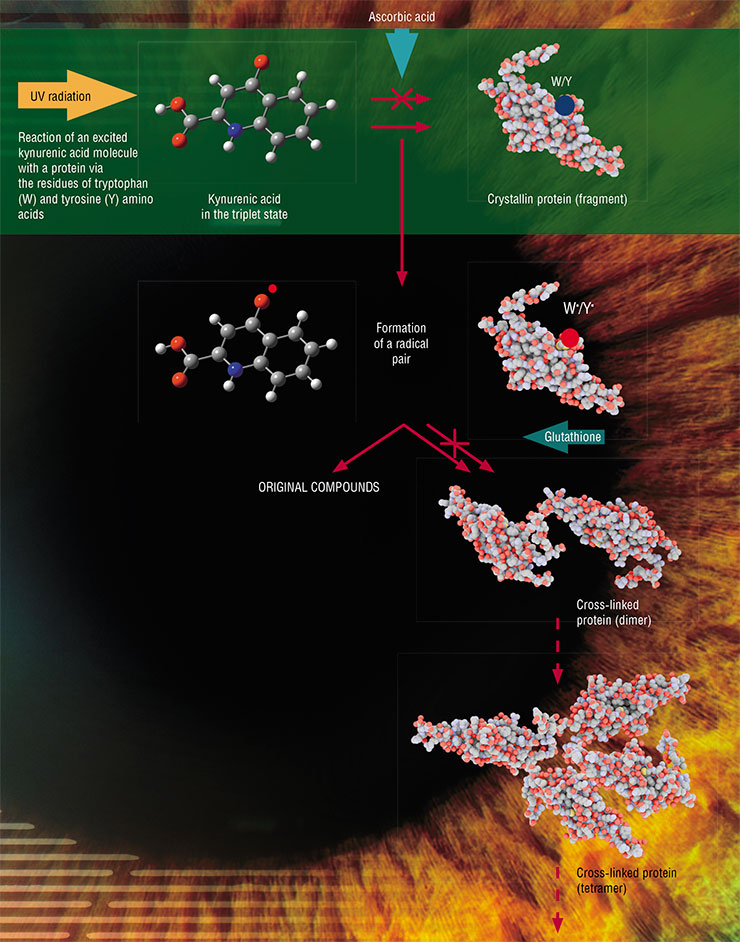
Kynurenic acid, one of the kynurenine derivatives, is a powerful photosensitizer. However, experiments with the prolonged irradiation of proteins in the presence of this compound yielded unexpected results. Under UV-A light, kynurenic acid forms triplet states with almost 100 % efficiency (one molecule per one quantum of light), but only a small fraction of subsequent reactions leads to protein modifications (Sherin et al., 2016).
It turned out that in the triplet state, kynurenic acid readily withdraws an electron from proteins to form free radicals and no less quickly returns it back when they meet again. Consequently, the formed radicals react via fast reverse reactions leading to the formation of original substances, rather than reactions leading to protein damage (Sormacheva et al., 2020). Nevertheless, a protein with a radical “suffers” from having an unpaired electron, which it tries to share with a similar partner to form a complete electron pair. As a result, two protein molecules form a strong and tight covalent bond, linking (“stitching”) together forever.
This protein-linking process goes quite slowly because the protein molecules are folded in large globules and they need time to find radical centers on each other’s surfaces. But the small and mobile kynurenic acid molecule can do this much faster by neutralizing the proteins’ radical centers. Nevertheless, some proteins bind to each other, forming globules of two, three, or more molecules. At some stage, they can lose water solubility and precipitate. This is how light-scattering areas appear in the eye lens and a cataract starts to develop.
These experiments yielded another interesting result by clarifying the role of molecular oxygen in photochemical reactions (Sormacheva et al., 2020). We found that the kynurenic acid radical rapidly transfers the excessive electron to oxygen forming a superoxide anion and the kynurenic acid in the original state. Although the superoxide anion is widely known as a precursor for dangerous reactive forms of oxygen, its formation promotes the return of kynurenic acid and proteins to their original states. These results demonstrated an amazing effect: oxygen protects proteins from irreversible modifications in amino acids.
It is known that the human lens contains high concentrations of antioxidants: glutathione and ascorbic acid. Photolysis of kynurenic acid with protein in the presence of these compounds showed that both antioxidants indeed successfully prevent protein modifications and can protect the lens from photoinduced damage (Sherin et al., 2016).
However, they achieve this end in different ways. Ascorbic acid acts as an effective “quencher” of triplet states, thus preventing excited kynurenine molecules from reacting with protein molecules. Glutathione, in turn, is a member of second-line defence as it is able to intercept free radicals, restore them to their original state, and prevent the linking of protein molecules via radical reactions.
It should be noted that the efficiency (or, in the language of photochemistry, the quantum yields) of reactions leading to the photodamage of lens proteins is very low due to two reasons. First, the yield of triplet states for most kynurenines does not exceed 1 % (Tsentalovich et al., 2008). Second, the most photochemically active kynurenic acid under oxygen-free conditions induces total protein decay at the level of only 1–2 % of the absorbed light quanta (Sherin et al., 2016; Savina et al., 2020). Thus, the overall efficiency of photoinduced radical reactions of kynurenines in the lens does not exceed 0.01 %. Furthermore, natural antioxidants, i. e., glutathione and ascorbic acid, greatly reduce the efficiency of these reactions by intercepting reactive particles and almost completely preventing photoinduced protein modifications.
To preserve the integrity of the eye lens, nature employs a unique cascade of processes for the transformation of the energy of hazardous UV radiation into heat. As a result, eye tissues remain effectively protected from harmful solar radiation in young and middle age. With ageing, the transport of small molecules in the lens becomes less and less efficient, which leads to the accumulation of degradation products and a slowdown in the supply of nutrients, including antioxidants. As a result, protein modifications go faster, and the accumulation of these irreversible changes may trigger the development of cataracts.
However, cataracts do not come as an inevitable consequence of ageing, and the lenses of many people remain transparent even at very old age. The most common questions that are asked on this topic are: “Why does the lens of most people remain transparent until the age of 50, and then it suddenly begins to cloud?” and “Is there anything that we can do right now to slow down the development of cataracts?”
We believe that the development of cataracts is primarily due to the weakening of the lens's natural antioxidant defence rather than the long-term accumulation of modified proteins. This approach leads to rather trivial recommendations – the best prevention of cataracts is a healthy and active lifestyle to maintain the body’s defence systems in good condition.
Note that the human body cannot synthesize ascorbic acid, which plays an important role in protecting lens proteins. Therefore, it is very important to eat enough fresh fruits and other foods containing this antioxidant throughout the year. And since the content of UV filters decreases substantially in the lenses of older people, it is much more important for them than for young ones to use sunglasses in everyday life.
References
Bova L. M. Sweeney M. H., Jamie J. F., and Truscott R. J. Changes in Human Ocular UV Protection with Age // Invest. Ophthalmol. Vis. Sci. 2001. V. 42. N. 1. P. 200–205.
Korlimbinis A., Aquilina J. A., and Truscott R. J. W. Protein-bound and free UV filters in cataract lenses. The concentration of UV filters is much lower than in normal lenses // Exp. Eye Res. 2007. V. 85. P. 219–225.
Savina E. D. Tsentalovich Yu. P., Sherin P. S. UV-A induced damage to lysozyme via Type I photochemical reactions sensitized by kynurenic acid // Free Rad. Biol. Med. 2020. V. 152. P. 482–493.
Sherin P. S., Grilj J., Tsentalovich Yu. P., and Vauthey E. Ultrafast excited-state dynamics of kynurenine – a UV filter of the human eye // J. Phys. Chem. B. 2009. V. 113. P. 4953–4962.
Sherin P. S., Grilj J., Kopylova L. V. et al. Photophysics and photochemistry of UV filter kynurenine covalently attached to amino acids and to a model protein // J. Phys. Chem. B. 2010. V. 114. P. 11909–11919.
Sherin P. S., Zelentsova E. A., Sormacheva E. D. et al. Aggregation of -crystallins in kynurenic acid-sensitized UVA photolysis under anaerobic conditions // Phys. Chem. Chem. Phys. 2016. V. 18. P. 8827–8839.
Tsentalovich Yu. P., Snytnikova O. A., and Sagdeev R. Z., Photochemical and thermal reactions of kynurenines // Usp. Khim. 2008. V. 77:9. P. 844–853; Russian Chem. Reviews. 2008. V. 77:9. P. 789–797
R. van Heyningen. Fluorescent Glucoside in the Human Lens // Nature. 1971. N. 230. P. 393–394.
This work was supported by the Russian Science Foundation (project 18-73-10014)