NMR Method: Towards Sensitive Detection
In 2011, a new laboratory—the Laboratory of Magnetic Resonance—was organized at Novosibirsk State University with the financial support of the Russian Government. The director of the laboratory is Prof. R. Kaptein (Utrecht University, the Netherlands). The laboratory has developed new approaches to substantially improve the sensitivity of NMR spectroscopy and imaging and offer new opportunities for the study of fast chemical reactions in biological systems and protein-nucleic acid interactions and further development of medical applications such as NMR imaging of lungs, which is almost impossible by the traditional method
Currently, one of the most effective methods for analyzing the structure of compounds at the molecular level is based on nuclear magnetic resonance (NMR), which occurs in the interaction between magnetic nuclei and an external magnetic field. The phenomenon itself was officially discovered by the US scientists F. Bloch and E. M. Purcell in 1946, although it had been predicted theoretically. As regards electrons, magnetic resonance was observed in an experiment by the Russian researcher E. Zavoisky as early as in 1944.
The first NMR spectrometers were designed so that a sample placed in a constant magnetic field was continuously subjected to variable radio frequency exposure. Today, researchers use short radio frequency (RF) pulses to get a response from the spin system; multipulse NMR methods have also become very popular. Thus, it is now possible to obtain not only one-dimensional (i.e., one-frequency) NMR spectra but also two-, three-, and multidimensional ones. Analysis of these spectra allows one to collect a broad range of highly detailed information about molecules, including their orientation, spatial structure, intermolecular interactions, etc.
Many elementary particles have a nonzero spin (e.g., the spin of an electron or proton is 1/2); the spin of a deuterium nucleus is 1. The most convenient particles for NMR experiments are nuclei with a spin of 1/2, e.g., 1Н, 13С, or 15N. The “necessary” isotopes of some of the elements exist in nature in sufficient concentrations; others require the isotope enrichment of the sample.
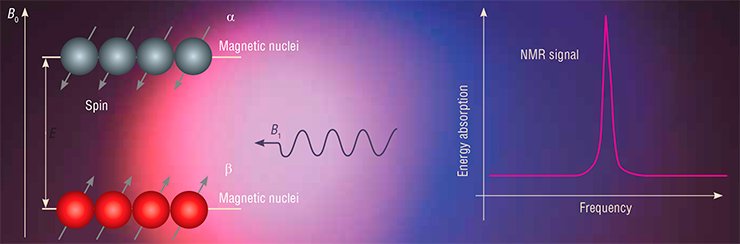
When an NMR spectrum is recorded, a sample is placed into a strong magnetic field: the stronger the field, the higher the sensitivity and spectral resolution. By the laws of quantum mechanics, a magnetic nucleus with a spin of 1/2 will be in one of the two states whereby the spin is directed either along or opposite to the field.
The splitting of energy spin levels in the magnetic field will be directly proportional to the field strength. Scientists often use the relationship between the energy E and frequency ν, which is given by the well-known Planck formula (E = hν), to measure the distance between the energy levels in units of frequency, not energy. For instance, in a magnetic field with an induction of 7 Tesla, the splitting of the energy levels of protons corresponds to a frequency of 300 MHz and that of 13С nuclei corresponds to approximately 75 MHz. Thus, the characteristic absorption frequencies fall into the radio frequency range of electromagnetic waves.
To observe the NMR effect, one needs to apply, in addition to a strong constant magnetic field, a variable radio frequency field with a frequency of E/h. Then the spins of the sample begin to resonate, or sense the influence of electromagnetic radiation, and the sample begins to absorb energy. In other cases, the sample remains “transparent” to the electromagnetic wave.
These developments have led to the wide use of NMR spectroscopy as a powerful research tool not only in inorganic chemistry but also in the study of biological macromolecules such as proteins and nucleic acids. It is difficult to overestimate the medical importance of magnetic resonance imaging (MRI) as a noninvasive diagnostic method. That is why there is a great demand for solutions enabling researchers to improve NMR sensitivity.
Toward nonequilibrium polarization
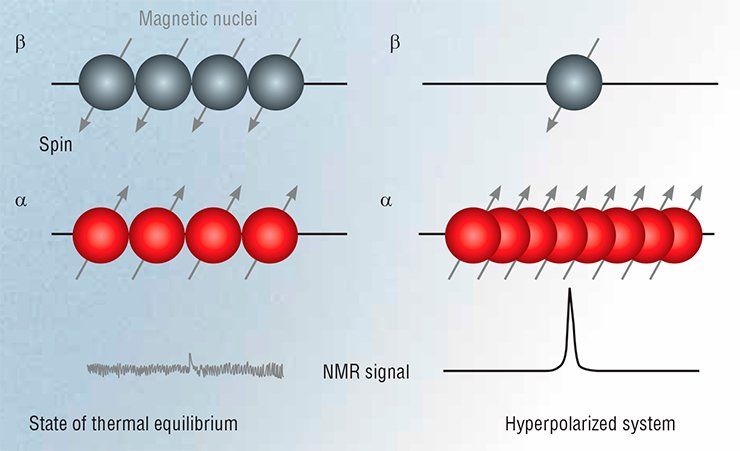
Although NMR spectroscopy offers a great many advantages, it has one major drawback, i.e., relatively low sensitivity. This is due, firstly, to difficulties associated with the detection of quanta of a low-energy electromagnetic wave, which are either absorbed or emitted by the spin system and, secondly, to the weak polarization of the spin system; the latter factor is discussed below in more detail.
The reason is that, even in the most powerful magnetic fields, the energy of resonant absorption is much lower than that of thermal motion of particles. As a result, under the equilibrium conditions, due to the Boltzmann energy distribution, the population of states for the spin along the field and opposite to the field is almost the same: the difference is 0.01% or less of the sum of the populations.
In other words, when a spin system interacts with an electromagnetic wave under normal conditions, it emits and absorbs almost the same number of quanta: in fact it is only 0.01% of all spins in a sample that contribute to the observed NMR signal! At the same time, if we somehow increase the degree of polarization of the spin system, the NMR signal will grow proportionally. The maximum possible increase in the signal will reflect a situation whereby all the spins are in the same state.
ROBERT KAPTEIN: A FOUNDER OF SPIN CHEMISTRY In 1969, while still a postgraduate student, the well-known Dutch scientist Prof. R. Kaptein proposed an explanation for the effect of chemically induced dynamic nuclear polarization, which is, perhaps, the most visible manifestation of the influence of spin degrees of freedom on the reactivity of molecules. This scientific contribution made Prof. Kaptein one of the founders of a new field in chemistry.Later on, his research interests shifted toward biological applications of NMR spectroscopy, and in 1975 Kaptein became the head of the national center for NMR studies of biomolecules at the University of Groningen (the Netherlands), which had purchased an NMR spectrometer operating at a frequency of 360 MHz, the highest frequency at that time. Later on, the scientist began to work at the University of Utrecht, where his research group had access to the best research facilities. Today the University of Utrecht has nine NMR spectrometers covering a range of electromagnetic frequencies from 360 to 900 MHz. Moreover, there is a powerful computing cluster and a chemical laboratory for the synthesis of proteins, including isotopically substituted ones.
Already in the 1990s, Kaptein’s group became not only a national research center but one of the world’s leading bio-NMR laboratories and one of the three NMR laboratories funded by the EU. The researchers made a great progress in the study of proteins involved in regulatory genetic processes; in particular, they identified the structures of gene regulatory proteins, glucocorticoid receptors, etc., and investigated the synthesis control system and specific action of lactose repressor protein.
The key to organizing a laboratory in Novosibirsk within the government mega-grant program was Kaptein’s long-term close relations with the Novosibirsk Science Center, in particular, with Academician R. Z. Sagdeev, the present director of the SB RAS International Tomographic Center (ITC, Novosibirsk). They first met in the 1970s, when Sagdeev was a head of laboratory at the SB RAS Institute of Chemical Kinetics and Combustion (Novosibirsk). The ITC now has a strong research team, which is working actively in the fields of NMR spectroscopy, NMR imaging, spin chemistry, and chemical kinetics. Together with the staff of the Laboratory of Biopolymer Modification, SB RAS Institute of Chemical Biology and Fundamental Medicine (Novosibirsk), they form the backbone of the Laboratory of Magnetic Resonance (Novosibirsk State University), led by R. Kaptein.
Even a relatively small shift in the state of equilibrium polarization may greatly expand the possibilities of NMR methods without needing to repeat the usual experiments, which are rather time-consuming.
 Although today there is a range of methods for creating spin hyperpolarization, here we will mention only two of them, which use chemical reactions to create hyperpolarized spins, i.e., chemically induced dynamic nuclear polarization (CIDNP) and parahydrogen induced polarization (PHIP).
Although today there is a range of methods for creating spin hyperpolarization, here we will mention only two of them, which use chemical reactions to create hyperpolarized spins, i.e., chemically induced dynamic nuclear polarization (CIDNP) and parahydrogen induced polarization (PHIP).
Nuclear polarization methods
In the CIDNP method, nuclear spins are polarized due to the reaction of a pair of radicals (particles with an unpaired electron) in a constant magnetic field. The reaction is effective if the electron spins are in a strictly defined state, i.e., antiparallel. In all other cases, the first phase of the reaction is electron spin conversion, the rate of which is determined by the so-called hyperfine interaction between the electron and nuclear spins. As a consequence, the reaction products are enriched with certain spin states of magnetic nuclei, i.e., are nonequilibrium-polarized (Salikhov, Molin, Sagdeev, et al., 1984).
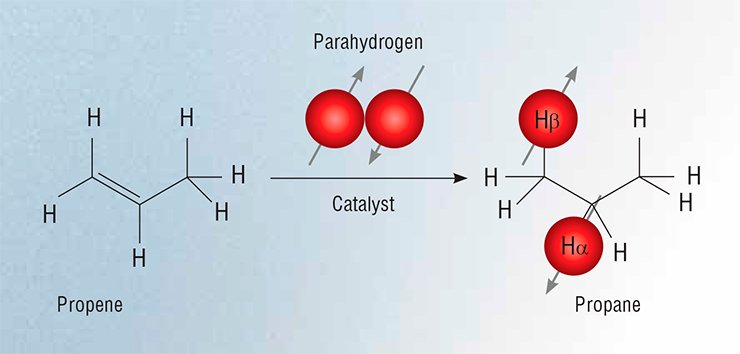
In the PHIP method, nuclear spins are polarized using parahydrogen molecules. In fact the nuclear pair of an Н2 molecule can be in two different spin states: the triplet state if the nuclear spins are parallel (orthohydrogen) and the singlet state if they are anti-parallel (parahydrogen). Under equilibrium conditions at room temperature, ortho- and parahydrogen exist in the gaseous phase in a 3:1 ratio; however, this ratio can be readily shifted toward parahydrogen to polarize the spin system. But there is one caveat to this. By the laws of quantum mechanics (the Pauli exclusion principle), the singlet state of two spins cannot be influenced by resonance radiation; therefore, the enrichment of molecular hydrogen with parahydrogen will only reduce the signal from the Н2 molecule.
The problem can be circumvented by using catalytic hydrogenation to add two hydrogen atoms to the substrate molecule so that the two of them would have different chemical environments. This helps to obtain high-intensity NMR signals for the reaction product (Natterer and Bargon, 1997).
Thus, nonequilibrium spin polarization can be created during a chemical reaction. However, this circumstance can be considered from a different angle: spin hyperpolarization carries information on the chemical processes that have led to its formation. Fast reactions involving radical particles play an important part in many chemical and biological processes, and the CIDNP method is an extremely useful and, in some cases, the only tool to explore these processes.
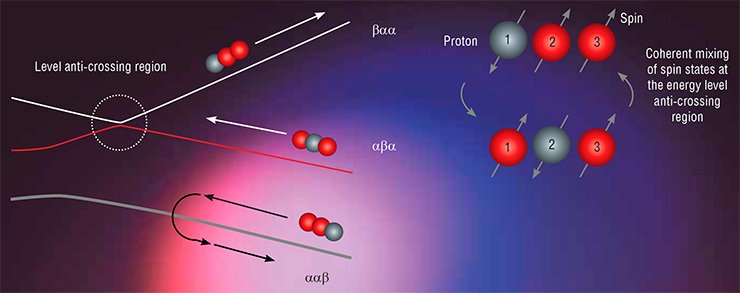
Within the mega-grant project, there has been substantial progress in developing methods for the transfer of non-equilibrium spin polarization, which are based on the concept of the so-called level anti-crossings, or avoided level crossings. Already in 1929, J. Wigner and J. von Neumann found that this phenomenon occurs in a quantum mechanical system when its energy levels coincide (cross) in the first-order approximation. However, if there is a perturbation (in our case, a spin-spin interaction) mixing the original states, the condition for level crossing cannot be satisfied: the levels are pushed apart (the case of anti-crossing). Importantly, it is at the regions of anti-crossings that the system’s original states can be mixed effectively; therefore, it is possible to change these states in a controllable way, i.e., transfer spin polarization.
Novosibirsk researchers have designed several schemes of experiments on the transfer of spin hyperpolarization. To satisfy the conditions for level crossing, they either switched the external magnetic field (to this end they designed a unique NMR setup with field switching) or applied RF fields with properly chosen parameters. The experimental results allowed them to formulate fairly simple rules for the most effective transfer of polarization and determine its pathway (Ivanov, Pravdivtsev, Yurkovskaya et al., 2014).
Chemically induced dynamic nuclear polarization is, as a rule, sustained in reaction products for a few seconds and carries information on particles reacting in the range from nano- to microseconds. In this case, the NMR spectrum allows one to identify signals from individual atoms in the molecule and analyze their polarizations, which helps to reveal fine details in the structure of short-living radicals and determine the rates and pathways of radical reactions.
An example is the study carried out by Novosibirsk researchers on the intramolecular transfer of the electron in oxidized dipeptides containing such amino acids as tyrosine, tryptophan, and glycine. Due to the short lifetime of tyrosine and tryptophan radicals, the traditional methods (e.g., recording magnetic resonance signals from their unpaired electrons) are, as a rule, ineffective in the study of their reactions. On the other hand, optical spectroscopy methods, although they have a good time resolution, cannot distinguish the forms of radical particles.
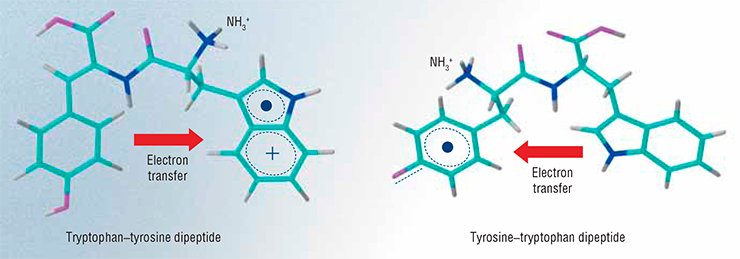 The researchers applied CIDNP to discover that the rate of electron transfer from the remaining part of tyrosine to the tryptophan radical changes by two orders of magnitude, depending on the charge and position of the terminal amino group. However, this difference for the pH-dependent rate of electron transfer in the reverse direction is not so great: the maximum and minimum constants of the rate of transfer at a fixed pH differ only by a factor of four. These results are explained by the greater destabilizing effect of the positive charge of the amino group on the tryptophan radical than on the tyrosine one. Moreover, the presence of glycine as a spacer reduces the difference between the redox potentials of tryptophan and tyrosine. Consequently, all these factors may affect the choice of the path of electron migration in protein molecules that are in certain functional states, i.e., affect the enzyme action.
The researchers applied CIDNP to discover that the rate of electron transfer from the remaining part of tyrosine to the tryptophan radical changes by two orders of magnitude, depending on the charge and position of the terminal amino group. However, this difference for the pH-dependent rate of electron transfer in the reverse direction is not so great: the maximum and minimum constants of the rate of transfer at a fixed pH differ only by a factor of four. These results are explained by the greater destabilizing effect of the positive charge of the amino group on the tryptophan radical than on the tyrosine one. Moreover, the presence of glycine as a spacer reduces the difference between the redox potentials of tryptophan and tyrosine. Consequently, all these factors may affect the choice of the path of electron migration in protein molecules that are in certain functional states, i.e., affect the enzyme action.
Propane based MRI
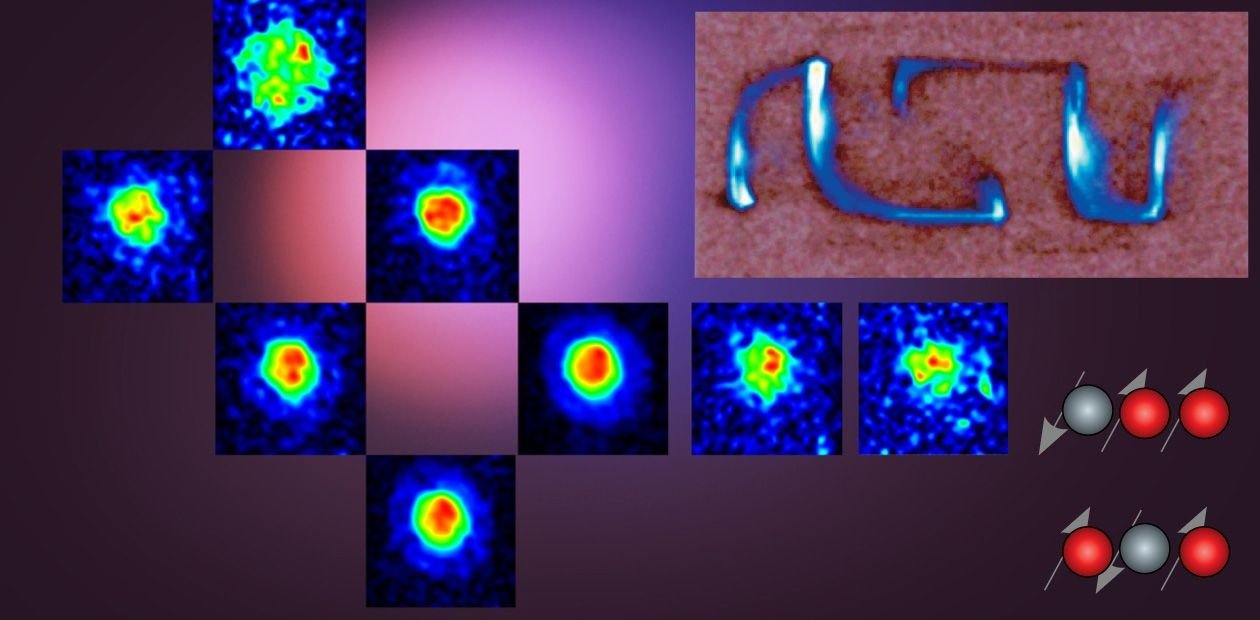
A major breakthrough of the Novosibirsk researchers was the development of an approach for using PHIP to obtain polarized “non-noble” gases and applying them in MRI.
Speaking about medical MRI, it should be noted that MRI diagnostics has traditionally focused on NMR signals from protons, which are abundant in the tissues of living organisms in the composition of water and fat. Lungs, however, have a low concentration of hydrogen atoms since a large part of their volume is air, which results in a very low sensitivity of the traditional MRI.
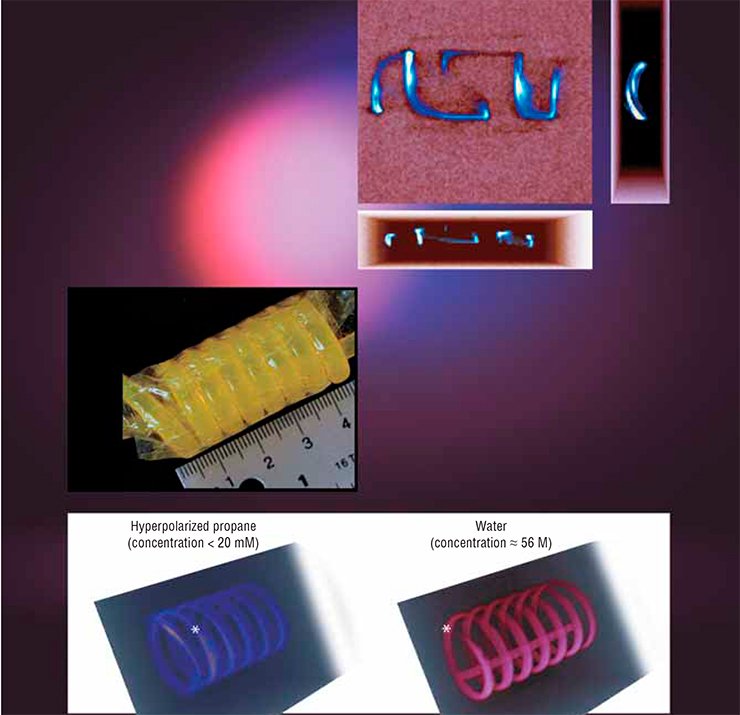 In this case, the MRI signal is usually amplified by employing contrast agents such as the noble gases 129Хе and 3Не. However, bringing these gases to a hyperpolarized state requires an expensive optical pumping procedure, but there are fewer than a hundred facilities worldwide capable of doing that. It is also important that the majority of clinical MRI scanners are fundamentally unable to detect signals from helium and xenon nuclei. Therefore, the use of other hyperpolarized gases, such as propane, not only opens the way to the development of other applications but also makes the applied studies considerably less expensive.
In this case, the MRI signal is usually amplified by employing contrast agents such as the noble gases 129Хе and 3Не. However, bringing these gases to a hyperpolarized state requires an expensive optical pumping procedure, but there are fewer than a hundred facilities worldwide capable of doing that. It is also important that the majority of clinical MRI scanners are fundamentally unable to detect signals from helium and xenon nuclei. Therefore, the use of other hyperpolarized gases, such as propane, not only opens the way to the development of other applications but also makes the applied studies considerably less expensive.
The Novosibirsk researchers applied successfully the above PHIP (parahydrogen induced polarization) method to obtain these hyperpolarized gaseous contrast agents, which were then used for the MRI visualization of various objects. For comparison, the researchers visualized the same objects filled with water, not gas. The propane signal proved to be lower than that of water only by a factor of 1.5 to 2.0 although the concentration of propane molecules was lower by three orders of magnitude.
Moreover, using a clinical MRI scanner with a strong (4.7 Tesla) magnetic field, the researchers managed to obtain three-dimensional NMR images with high spatial resolution for objects filled with polarized propane. This accomplishment, which has no counterparts in the world practice, suggests that propane obtained by parahydrogen hydrogenation can be successfully used for three-dimensional MRI visualization of human lungs, with this method being much more informative than photofluorography.
 There were also experiments with MRI of gases in low magnetic fields. It was found that the signal from hyperpolarized propane was even stronger than that of water! The reason is that gas hyperpolarization achieved through catalytic hydrogenation does not depend on the value of the applied magnetic field while the water signal is linearly dependent on that field. Therefore, it is expedient to use weak fields for MRI based on hyperpolarized gases. Besides, low-field MRI scanners can be rather inexpensive since they do not require superconducting magnets.
There were also experiments with MRI of gases in low magnetic fields. It was found that the signal from hyperpolarized propane was even stronger than that of water! The reason is that gas hyperpolarization achieved through catalytic hydrogenation does not depend on the value of the applied magnetic field while the water signal is linearly dependent on that field. Therefore, it is expedient to use weak fields for MRI based on hyperpolarized gases. Besides, low-field MRI scanners can be rather inexpensive since they do not require superconducting magnets.
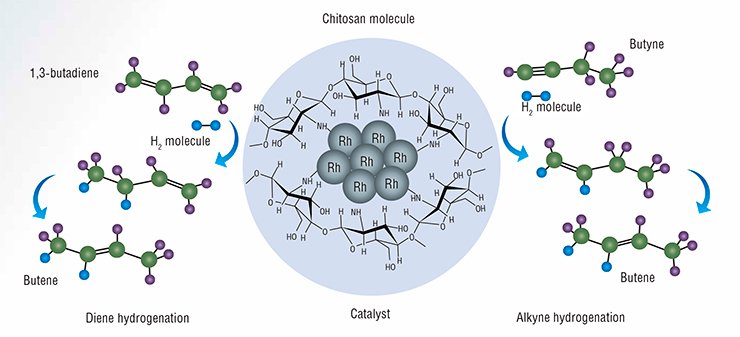
It is now possible to develop new methods for producing hyperpolarized gases owing to the development of fundamentally new catalytic systems allowing researchers to generate a continuous flow of polarized fluids. It is noteworthy that the International Tomography Center SB RAS (ITC, Novosibirsk) has been the world’s first research institution to demonstrate the possibility of observing PHIP effects in the use of heterogeneous catalysts for hydrogenation of unsaturated hydrocarbons both in the liquid and gaseous phase. Within this project, the ITC researchers also studied a new catalytic system in which chitosan, a natural polysaccharide, serves as a carrier for metal nanoparticles.
From DNA to cataract
It is known that cells of living organisms are constantly exposed to ionizing and ultraviolet radiation and to various chemicals mutagens; moreover, cell metabolism creates reactive oxygen species. All this primarily affects the genomic DNA by damaging it and provoking cancers and various cardiovascular, neurodegenerative, and autoimmune diseases.
Cells protect genetic information by means of DNA repair systems. The Novosibirsk researchers used NMR spectroscopy to investigate DNA structures with different defects and DNA complexes with repair enzymes. Traditionally, these studies are carried out using X-ray spectroscopy of DNA and protein–nucleic acid complexes in crystalline form. However, the structures of biological objects in solutions may be seriously different from those in crystals; therefore, NMR studies are very important for understanding the nature of the highly specific action of repair enzymes on DNA.
 A major biological application of NMR is the detection and analysis of photochemical and biochemical processes in the lens inside the eye, which are responsible for normal aging and cataract development.
A major biological application of NMR is the detection and analysis of photochemical and biochemical processes in the lens inside the eye, which are responsible for normal aging and cataract development.
Currently, cataract (clouding of the eye lens) develops in more than a half of people over 65 and is the most widespread cause of visual impairments in elderly people. Moreover, clouding of the lens is often regarded as an inevitable manifestation of aging. Today this disease is, as a rule, diagnosed at the irreversible stage, when therapeutic intervention is ineffective. Therefore, the only way to restore vision is to remove the eye lens and put an artificial one. The lack of knowledge about the mechanisms of cataract development impedes the search for medications that could halt the progress of the disease and restore the transparency of the eye lens.
The Novosibirsk researchers showed that cataract development is accompanied by a decrease in the quantity of lens molecules that act as UV filters and prevent the damaging of light sensitive cells in the retina (rods and cones). This process is also accompanied by an increase in the content of tryptophan, an amino acid used as material for the enzymatic production of molecular UV filters.
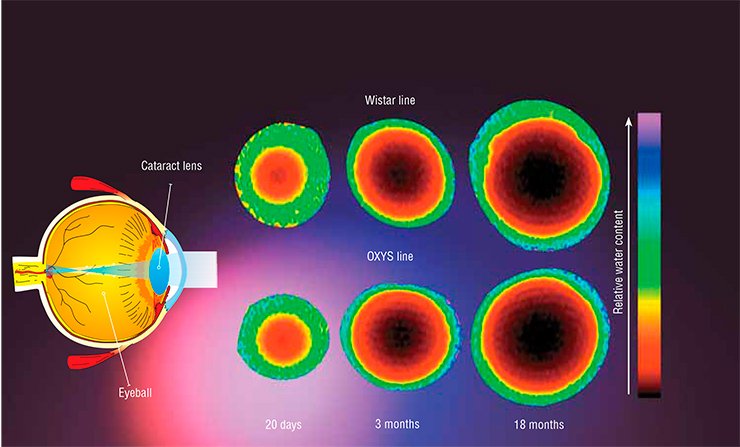 Another important aspect of cataract development is the numerous modifications of lens proteins, which may lead to a loss of solubility and, hence, to precipitation. The Novosibirsk researchers studied prematurely aging OXYS rates, which were developed at the Institute of Cytology and Genetics SB RAS (Novosibirsk), to identify the types of change in the chemical modifications of proteins. Their results are important for understanding the chemical reactions leading to these pathological changes.
Another important aspect of cataract development is the numerous modifications of lens proteins, which may lead to a loss of solubility and, hence, to precipitation. The Novosibirsk researchers studied prematurely aging OXYS rates, which were developed at the Institute of Cytology and Genetics SB RAS (Novosibirsk), to identify the types of change in the chemical modifications of proteins. Their results are important for understanding the chemical reactions leading to these pathological changes.
MRI studies on the transport of small molecules in the eye lens of rats yielded unexpected results: it turned out that cataract development does not lead to a change in the content and diffusion rate of water molecules. The change in the content of other compounds such as amino acids and molecular UV filters appears to be due to the existence of a barrier to large-sized molecules. Today the nature of this phenomenon is still unknown.
Further studies on the changes in the compositions of proteins and small molecules in the lens inside the human eye in the case of normal aging and cataract development will provide recommendations for early diagnosis, prevention, and treatment of this widespread disease.
The most important part of an NMR spectrometer is an electromagnet creating a constant magnetic field due to the electric current in the solenoid. The greater the current, the stronger the field; however, very high values of the current may lead to the melting of the solenoid. Therefore, high field NMR spectrometers use superconducting magnets because the electrical resistance of a superconducting wire is zero. The superconducting state can be obtained only at very low temperatures (those of liquid helium). These magnets have an advantage that they themselves consume no energy: after starting the magnet, the current runs through the superconducting wire with virtually no losses for many years.The design of the superconducting magnet is based on the matryoshka (nested-doll) principle. The central vacuum chamber contains a solenoid enveloped in a shell of liquid helium. The next shell is filled with liquid nitrogen at a temperature of –196°С. The device is insulated from the external environment by one more vacuum shell. This system is able to keep the superconducting magnet at a low temperature for a very long time; however, maintaining this system is not an easy task. This explains the technical challenges in the manufacture of these magnets and their high price.
The project, which was supported by the government mega-grant program, is now complete, but the work of the multidisciplinary research team is not over. The studies planned for 2014—2016 are supported by the Russian Science Foundation (RSF), which has provided 60 million rubles. The ITC has also received funding from the RSF for creating a new laboratory on MRI studies of brain, including with the use of new hyperpolarization methods.
REPORT IN FIGURES The mega-grant funding was used to purchase a number of expensive research instruments including, first of all, a modern NMR spectrometer with a magnetic field of 16.4 Tesla (the working frequency for proton resonance is 700 MHz) for studying biomolecules. The spectrometer has a set of probes to measure signals from different magnetic nuclei. With the assistance of R. Kaptein, the Bruker company designed the Prodigy cryoprobe for this frequency; the sensitivity of these probes is greater than that of standard ones by a factor of three to four. The use of Prodigy should cut the time needed to obtain multidimensional NMR spectra of biomacromolecules by 90%. The mega-grant money also went to purchase other laboratory equipment such as a device to prepare thin sections of samples at ultralow temperatures and a parahydrogen generator.With the financial support of the Alexander von Humboldt Foundation (Germany), a conference was organized on magnetic resonance applications. The Novosibirsk researchers also were among the participants of a COST (the Cooperation in Science and Technology initiative funded within the 7th Framework Programme of the European Union) project on the development and application of spin hyperpolarization methods in NMR spectroscopy and imaging.
During the project period, the participants published 45 papers in highly rated science journals and designed lecture courses on NMR spectroscopy, including on NMR spectroscopy of biomolecules. Two doctor-of-science (the highest academic degree in Russia) and candidate-of-science dissertations were defended. Young academics working at the laboratory (12 people) had an opportunity to do an internship at leading research centers of the Netherlands, Germany, France, and the United States.
References
Ivanov K. L., Pravdivtsev A. N., Yurkovskaya A. V., Vieth H.-M., Kaptein R. The role of level anti-crossings in nuclear spin hyperpolarization. Prog. NMR Spectrosc. 2014. V. 81. P. 1—36.
Natterer J., Bargon J., Parahydrogen induced polarization. Prog. Nucl. Magn. Reson. Spectr. 1997. V. 31. P. 293—315.
Salikhov K. M., Molin Y. N., Sagdeev R. Z., Buchachenko A. L. Spin Polarization and Magnetic Effects in Chemical Reactions. Elsevier, Amsterdam, 1984.
This work was supported by the Government of the Russian Federation, project no. 11.G34.31.004, and the Russian Science Foundation project nos. 14-13-01053, 14-14-00056, 14-14-00063, and 14-13-00445.
Translated by A. Kobkova