Metabolome – the molecular mirror of life
In our bodies, proteins, the products of activation of certain genes, are not the only substances that constantly change in their quantity and composition; there are also simpler organic molecules that originate from various metabolic processes or entering it from the environment. Such molecular “profiles” contain unique information that can be used for a variety of applied purposes. Researchers from Novosibirsk have used this approach to discover the reasons underlying the development of cataracts, find promising prebiotics and tumor biomarkers, and help forensic experts with a problem crucial in solving crimes
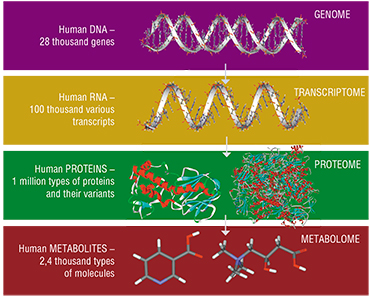 The second half of the XX century was marked by a boom of molecular biology, and genetics in particular. The successful implementation of one of the most ambitious project in the history of science, the Human Genome Project, is a vivid example. These events gave rise to a whole group of the so-called “postgenomic” sciences, not widely known to the public, dealing with the study of molecular products of realization of hereditary information in the organism.
The second half of the XX century was marked by a boom of molecular biology, and genetics in particular. The successful implementation of one of the most ambitious project in the history of science, the Human Genome Project, is a vivid example. These events gave rise to a whole group of the so-called “postgenomic” sciences, not widely known to the public, dealing with the study of molecular products of realization of hereditary information in the organism.
Metabolomics, the youngest of all postgenomic sciences, studies the metabolome – the combination of all small (i. e. with a molecular mass of less than 1kDA*) molecules in the body or a particular organ, tissue, or cell. Metabolites include amino acids, organic acids, sugars, nucleotides and other classes of organic compounds.
Metabolomic analysis is the key to understanding various dynamic processes occurring in the body. Under normal conditions, the concentrations of metabolites in a tissue or a body fluid depend on their role in metabolic processes, and, as a rule, change insignificantly. However, disease can cause drastic changes in the metabolomic profile of the affected tissue. Studying the dynamics of the composition and concentrations of metabolites can shed light on the molecular origins of various diseases, or at least help to reveal their biological markers.
Today, there are three main directions of metabolomic research. Qualitative metabolomics detects and identifies metabolites in tissues regardless of their concentration. This approach is useful on the initial stages of studies, as well as for a number of specialized applications (such as anti-doping laboratories). The bulk of research in metabolomics is carried out using the semi-qualitative approach, which compares experimental and control samples (for instance, pathological and healthy tissues). The most informative of the three, qualitative approach is also the most labor-intensive: it consists in measuring absolute values of metabolite concentration in the studied tissue.

Researchers from the Laboratory of Proteomics and Metabolomics of the SB RAS International Tomography Center are developing and studying the applications of methods of quantitative metabolomics to solve specific problems of biology and medicine. Among other things, this approach enabled the scientists to shed light on the origins of cataracts, discover promising intestinal prebiotics, isolate biomarkers of highly resistant brain tumors and succeed in solving an important forensic problem – determining the exact time of death.
How to avoid cataract
The lens lives up to its name – it is a biological optical lens. To fulfill its optical functions, it must be flexible, transparent, and have a high refraction coefficient. However, over a half of all people older than 65 worldwide suffer from age-related nuclear cataract, when the center of the lens becomes opaque; it is the leading cause of blindness.
The lens is mostly composed of long hexagonal fiber cells that lack nuclei or organelles and are filled with large amounts of special structural proteins called crystallins. However, this highly advanced structure is flawed – the fibers of the lens have almost no metabolic activity, meaning that its structural proteins are not being renewed. The cells themselves are never replaced by newer cells, either: the “real” cells responsible for the growth of the lens throughout the life are present only in its epithelial layer, which consists of a single layer of non-squamous cells. This means that at any moment in time, the center of the lens contains the very same fibers with which the person was born, and the same proteins.
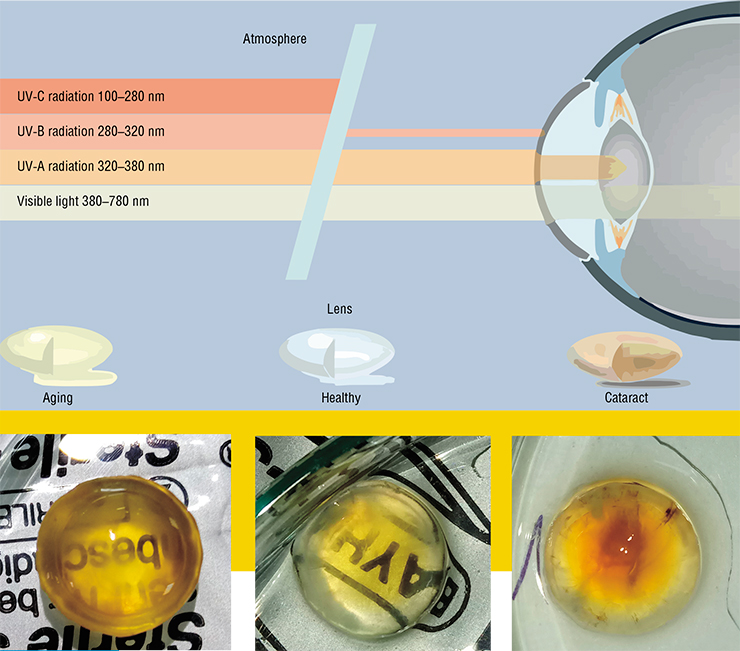
With age, the proteins of the lens get older, accumulating various modifications: they become yellow or even brown, lose solubility and aggregate. Sediments composed of large protein aggregates begin to scatter light, which is believed to cause cataracts. While the mechanism of this disease is understood, the primary reasons of its origin are still unclear.
One of the possible reasons is stress. We know that any biological tissue is prone to stress, be it oxidative stress, osmotic stress, or stress caused by solar radiation, etc. Since the majority of the regular cellular mechanisms do not operate in the lens cells, their only protection relies on metabolites (antioxidants, osmolytes, UV filters) that need to be synthesized somewhere and slowly diffuse towards the lens center. To understand the mechanisms of cataract development, we need to determine the origin of these compounds.
We know that the lens is immersed in intraocular fluid secreted by the ciliary body (part of the uvea, or the vascular layer of the eye, on which the lens is suspended). This liquid functions as blood – it supplies the lens with nutrients and removes waste. A comparison of metabolomics contents of serum and intraocular fluid showed that they are very similar, with one important exception: in the blood, the concentration of ascorbic acid, a crucial antioxidant, is hundreds of times lower.
 The thing is, in the course of evolution, humans and other primates lost a gene for one of the enzymes responsible for the synthesis of ascorbic acid. We compensate for this defect with ascorbic acid from food, primarily from fruit and root vegetables. However, the lens requires much more ascorbic acid than other tissues; consequently, the eye has specific pumps that take up the ascorbate from the blood and pumps it into the intraocular fluid. Further transfer of ascorbic acid into the lens occurs by means of regular diffusion.
The thing is, in the course of evolution, humans and other primates lost a gene for one of the enzymes responsible for the synthesis of ascorbic acid. We compensate for this defect with ascorbic acid from food, primarily from fruit and root vegetables. However, the lens requires much more ascorbic acid than other tissues; consequently, the eye has specific pumps that take up the ascorbate from the blood and pumps it into the intraocular fluid. Further transfer of ascorbic acid into the lens occurs by means of regular diffusion.
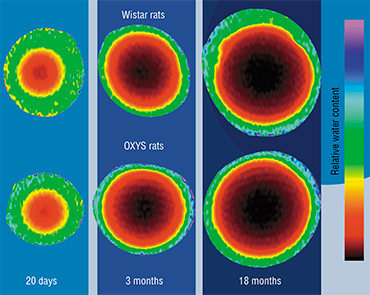
The comparison of metabolomic contents of intraocular fluid and the lens showed that there is another key antioxidant in the lens – a peptide called glutathione. Myo-inositol, a six-atom sugar alcohol, turned out to be responsible for sustaining intracellular pressure; a set of derivatives of kynurenine, a metabolic product of amino acid tryptophan, functions as UV filters. The concentration of all these compounds in the lens (with the exception of ascorbic acid) turned out to be many times higher than that in the intraocular fluid. The logical conclusion here is that the majority of “protective compounds” in the lens does not come from the outside, but is synthesized in the lens itself, or, more precisely, in its epithelial cells.
When researchers compared healthy and cataract-affected lenses of people of the same age, the latter were showed to contain much lower levels of osmolytes, antioxidants, and UV filters. This led to a hypothesis that cataract is caused by a decrease in the function of epithelial cells. This means that the optimal approach to cataract treatment and prevention may lie in restoring the normal function of epithelial cells, as opposed to “pumping” the lens with antioxidants. This is a much more complex task, and the solution will probably involve the use of cellular technologies. For example, the condition of an aging lens can be dramatically improved by implanting healthy epithelial cells.
From microbiome to glioma
Laboratory animals are an important part of the modern biomedicine: they serve as experimental models of human diseases. It is on animal models that researchers actively study the mechanisms underlying specific pathologies and test new methods of their prevention and treatment.
For instance, laboratory mice with congenital colitis and intestinal inflammation in the acute phase serve as models of patients with congenital and acquired forms of this disorder and with colorectal cancer. Similar models are used to search for efficient pro- and prebiotics, and to test methods of treatment by manipulating the intestinal microbiota.
One of the studies on metabolic profiling of tissue and serum of model animals conducted together with the SB RAS Institute of Cytology and Genetics and the Research Institute of Physiology and Medicine in Novosibirsk is dedicated to the interaction of the host organism and its intestinal microbiome based on model mice with congenital intestinal inflammation.
 This study was based on the observation that congenital colitis and colorectal cancer are often accompanied by a reduction in the thickness of the gel-like lining of the intestines, consisting primarily of mucin‑2, which is the key intestinal proteoglycan – a complex high-molecular-weight compound made up of a protein and a heteropolysaccharide. This mucous layer is the most favorable environment for symbiotic bacteria.
This study was based on the observation that congenital colitis and colorectal cancer are often accompanied by a reduction in the thickness of the gel-like lining of the intestines, consisting primarily of mucin‑2, which is the key intestinal proteoglycan – a complex high-molecular-weight compound made up of a protein and a heteropolysaccharide. This mucous layer is the most favorable environment for symbiotic bacteria.
The interaction of intestinal bacteria and mucin‑2 is based primarily on glycoside (“sugar”) chains, which contain monosaccharide residues. Researches have suggested that these components may play a definitive role in the structure of the intestinal microbiota, large intestine in particular, where mucin‑2 is synthesized in especially large volumes.
The influence of monosaccharides on the microbiota is studied using two mouse models of colon cancer: one caused by the active phase of chronic colitis and the other caused by a chemical agent. Researchers have revealed a positive effect of a monosaccharide – fucose, which normalizes the level of tryptophan in the serum and changes the structure of the intestinal bacterial communities, causing increased numbers of tryptophan-producing bacteria and a disappearance of behavioral anomalies accompanying an inflammatory process, observed in laboratory animals (Borisova et al., 2020).
Metabolomic profiling of tissues and serum of animal models of human diseases produces the knowledge necessary for understanding the mechanisms of various biochemical processes occurring in the organism both in the healthy and in various pathological states; eventually, this knowledge can be used in disease prevention and therapyThis is just the first step in the study of various promising qualities of sugars in relation to both normal and pathogenic microbiota of the host. In the future, such works will help to create prebiotics with predefined properties from different individual components.
Metabolomic analysis of model animal tissues can also help in developing diagnostic tools for glioma, which is the most widespread brain cancer in adult. These highly aggressive tumors are resistant to all currently available therapeutic treatment methods: glioma cells can quickly modify their metabolism and change, adapting to the environment.
By comparing the metabolomics composition of healthy animal tissues and tissues from animals with grafted human glioma cells, researchers discovered that the metabolomic profiles of healthy and pathological tissues differ by their concentration levels of a number of metabolites.

The results of all these studies also indicate that the dynamics of metabolic changes in the affected tissues is reflected in the blood serum metabolome. This means that blood can be used for metabolomic screening for both inflammatory and oncological diseases. Blood panels currently used in medical laboratories usually include determining the concentrations of just a few metabolites (such as glucose, creatinine, and uric acid). Increasing their number to several dozen will enable early diagnostics of a much broader range of diseases and, more importantly, it will significantly increase the precision and reliability of the diagnosis.
Holmes would never dream of it…
Modern detective stories and TV shows often picture the use of metabolomic methods in forensics, including solving one of the key forensic problems: determining the exact time of death, which is often crucial to solve a crime.
For this purpose, forensic experts traditionally measure the body temperature, evaluate postmortem lividity and rigor mortis, as well as the reaction of skeletal muscles to stimulants. Other markers of time of death include the stage of decomposition as well the presence of living organisms in the body, such as insect larvae. Nowadays, experts use various new “science-intensive” tools, such as studying isotope composition and evaluating the degradation of DNA and other molecules. However, these methods are neither precise nor reliable enough.
For a forensics expert, various biological fluids are the easiest to work with, as it simplifies working with the sample and saves time. The choice lies between blood, urine, synovial, epidural and ocular fluids. Urine tends to have very variable contents that strongly depend on various external factors, and synovial and epidural fluids are usually scarce and difficult to extract. In this sense, blood serum and the vitreous body – a gel-like filling of the eye, and intraocular fluid are more promising. The latter two are easy to obtain in pure form.

We know that after death, biological fluids undergo substantial changes defined by three major factors: the onset of anaerobic processes (i. e. chemical processes that take place without oxygen); cell lysis due to the failure of sodium-potassium proton pumps, which regulate intracellular pressure; and bacterial activity. These changes in the metabolomic composition of biological fluids provide an opportunity for establishing the postmortem interval. Metabolomics-wise, the optimal biomarkers of time of death would be metabolites with a monotonous (or, in the best case, linear) change of their concentration levels in body fluids for relatively long periods of time following death.
Researchers from Novosibirsk began the study of postmortem changes on model animals – rabbits (Zelentsova et al., 2016). Each animal was sampled for three biological fluids at various intervals after death, and concentrations of several dozen metabolites were measured. Based on the dynamics of these concentrations, the compounds were split into several groups: “concentration does not change”, “concentration grows”, “concentration falls” and “changes appear to be random”.
As the result, researchers found several substances (including choline, hypoxanthine, and glycerol) that demonstrated substantial (several ten-fold or hundred-fold) and nearly linear concentration increase with time. At the same time, serum contents were shown to change significantly more chaotically compared to the vitreous body and intraocular fluid. Consequently, it is the ocular fluids that show the highest promise for future research.
These results were justified using the samples of serum, intraocular fluid and vitreous body samples from people with different time of death. Samples were acquired from a specimen bank collected together with the Novosibirsk Regional Clinical Bureau of Forensics. Quantitative metabolomics analysis confirmed that intraocular fluid and the vitreous body were the most appropriate liquids, and that hypoxanthine and choline were the most promising biomarkers.
Ultimately, the data on changes in metabolite concentrations were used to develop a mathematical model that takes into account concentrations of several biomarkers at once, thus increasing the precision of estimating the time of death.
Even the few examples provided in this article clearly show that quantitative metabolomics can be used for a wide range of biological objects – from cell cultures to human tissues, and has been successively applied in solving various biological and medical problems.
In essence, this discipline is still in its infancy, however, there are reasons to believe that in the nearest future, metabolomics tissue profiling will be used not only to diagnose human diseases, but to select the optimal treatment schemes.
* 1 kDa =103 Da (Dalton – a unit of atomic mass equal to 1/12 of a free resting carbon atom 12C in the ground state)
References
Borisova M. A., Snytnikova O. A., Litvinova E. A., et al. Fucose ameliorates tryptophan metabolism and behavioral abnormalities in a mouse model of chronic colitis // Nutrients. 2020. V. 12. P. 445.
Snytnikova O. A., Khlichkina A. A., Yanshole L. V., et al. Metabolomics of the human aqueous humor // Metabolomics. 2017. V. 13(1). P. 5.
Tamara S. O., Yanshole L. V., Yanshole V. V., et al. Spatial distribution of metabolites in the human lens // Exp. Eye Res. 2016. V. 143. P. 68–74.
Tsentalovich Yu. P., Verkhovod T. D., Yanshole V. V., et al. Metabolomic composition of normal aged and cataractous human lenses // Exp. Eye Res. 2015. V. 134. P. 15–23.
Yanshole V. V., Yanshole L. V., Snytnikova O. A., and Tsentalovich Yu. P. Quantitative metabolomic analysis of changes in the lens and aqueous humor under development of age-related nuclear cataract // Metabolomics. 2019. V. 15(3). P. 29.
Zelentsova E. A., Yanshole L. V., Snytnikova O. A., et al. Post-mortem changes in the metabolomic compositions of rabbit blood, aqueous and vitreous humors // Metabolomics. 2016. V. 12. N. 11. P. 172.
The research was supported by RFBR (grants #18-29-13023, 18-33-20097 and 18-34-00137)