Mendeleev: On a Path to the Law
On the History of Discovery of the Periodic Law
Dmitri Mendeleev commenced his first paper on this law by stating that a “systematic arrangement of the elements has been faced, throughout the history of our science, with numerous vicissitudes.” That is true. However, Mendeleev did not mention one thing—the scientific community regarded the problem of a “systematic arrangement of the elements” as ultimately marginal or even beneath the notice of a serious scholar. For example, when one of Mendeleev’s forerunners, John Newlands, presented his classification of the elements (the so-called Law of Octaves) at a meeting of the Chemical Society of London, he was asked whether he had tried arranging the elements alphabetically since “any arrangement would present occasional coincidences” (Newlands, 1866, p. 113). Thus, Mendeleev took up a problem that was considered, in his days, as a subject of no importance, even of ridicule. Nevertheless, he decided to put serious effort into classification of the elements
 On October 18 (according to the Julian calendar), 1867, Dmitri Ivanovich Mendeleev, then a 33-year old professor at St. Petersburg University, was transferred from the Department of Technical Chemistry to the Department of Chemistry and promoted to full professor. In the same October, he began lecturing on inorganic chemistry to first-year students of the Physics and Mathematics Faculty. He kept on with this lecture course until his resignation from the university in 1890. Naturally, he needed a textbook for his students but found nothing adequate and decided to write a textbook of his own, which he entitled Principles of Chemistry (originally, he envisioned a title Principles of Chemistry, or a Universally Comprehensible and Intelligible Account of Inorganic Chemistry, Its Theory and Applications).
On October 18 (according to the Julian calendar), 1867, Dmitri Ivanovich Mendeleev, then a 33-year old professor at St. Petersburg University, was transferred from the Department of Technical Chemistry to the Department of Chemistry and promoted to full professor. In the same October, he began lecturing on inorganic chemistry to first-year students of the Physics and Mathematics Faculty. He kept on with this lecture course until his resignation from the university in 1890. Naturally, he needed a textbook for his students but found nothing adequate and decided to write a textbook of his own, which he entitled Principles of Chemistry (originally, he envisioned a title Principles of Chemistry, or a Universally Comprehensible and Intelligible Account of Inorganic Chemistry, Its Theory and Applications).
Mendeleev himself recounted, “I began to write when I took up lecturing on inorganic chemistry after Voskresensky at the University and, having looked through all the books, found nothing to recommend to my students. Many of my friends, too, forced me to write, for example, Florinsky, Borodin. While writing, I did learn a lot” (Dmitri Mendeleev’s Archive, vol. 1, 1951, p. 52). That is Mendeleev’s version of the story. However, there was another important reason that compelled Mendeleev to plod away at writing a “thick” textbook—and that was money.
By that time he had set out to renovate his recently purchased Boblovo estate in Tver Governorate, where he intended to create an exemplary land holding and conduct agricultural experiments. Moreover, he already was a family man and a father of a son, and the second child was soon to be born. The fee for the textbook, which could be republished, could give him a good side income. As Mendeleev sаid, “since I published it myself, I also made money from it, and later the book became my main source of side income—from new editions” (Ibid., p. 53). It should be noted, however, that Mendeleev forgot to mention that the university paid him a monetary allowance both for the first and second editions of his Principles.
Dmitri Mendeleev was a pioneer of Russian metrology. He attached great importance to the accuracy of measurements, even in his student days. “Science begins where one begins to measure,” he said, “exact science is unthinkable without measure.” Mendeleev designed and made instruments for his experiments either himself or ordered them from the best craftsmen. He believed that the scales are a “prototype of all precision instruments.” The scientist paid special attention to the accuracy of weighing, considering it the most effective type of measurements in science“…My composition,” Mendeleev wrote, “is not a textbook but rather an account of the entire corpus of my concepts and visions, some of which later became part of my memoirs, which underwent many reeditions. I myself do not stick to it when I deliver my lectures” (Mendeleev, 1876, p. 4).
The first issue of the Principles appeared in the late May or early June of 1868. In the summer of the same year, he was writing the second issue of the textbook, which he finished in March 1869. It was while working on the Principles that Mendeleev discovered the Periodic Law.
First attempt
The history of discovery of the Periodic Law and development of the Periodic System is complex and inextricable; thus, in what follows I will only outline the major landmarks on Mendeleev’s path to his major life achievement. I will begin with a testimony of his own:
“My first attempt in this regard was as follows: I chose bodies with the smallest atomic weight and arranged them in the order of their atomic weights. It turned out as if there was a period in the properties of primary bodies; even in terms of atomicity, the elements follow one after another in an arithmetic sequence of their shares:

While still examining these light elements (with atomic weights of 1 to 40), Mendeleev arrived at crucial assumptions:
(1) “What if the elements’ properties manifest themselves in their atomic weight? Is it possible to build a system upon it?” (Ibid., p. 18).
(2) When the elements are arranged in a system by their atomic weights, one can see “as if a period in their properties.” Thus, even if he did not—as of yet!—propose a complete formulation of the Periodic Law, he at least grasped its essence, i. e., the periodic nature of the changes in the elements’ properties with the change in their atomic weights, and all of his subsequent efforts were aimed at verifying this—as of yet—hypothesis.
(3) Is it possible to build a system of elements from structural units of the following form:

In other words, Mendeleev decided to build a system of elements by stacking fragments of type (1) so that the atomic weights increased from top to bottom and left to right.
John Newlands (1837—1898) was a British physicist and chemist. In 1864, he published a table where he arranged all the known elements in the increasing order of their atomic weights using Cannizzaro’s data. Newlands numbered the elements, compared their numbers with their properties and, noting that elements with similar properties recur regularly, concluded that the eighth element from a given element is a kind of repetition of the first one, like the eighth note of the octave in music. Although he did not use the term periodicity, he essentially revealed a periodic change in the properties of the elements“Haloids [halogens] and alkali metals,” wrote Mendeleev in Principles of Chemistry, “are in a sense the extreme elements; all the rest are in fact either metals, which tend to a certain degree to alkali metals both in the ability to yield salts and in the absence of hydrogen compounds [metal hydrides had not been discovered yet.—I.D.], but they are not so energetic as alkali metals. …Finally, there is a category of elements, such as carbon and nitrogen [here he meant substances that are not compounds.—I.D.], which show no aggressive manifestations of either metallic or haloid properties and thus occupy the interval between the two aforementioned categories of primary bodies. Obviously, this kind of primary bodies constitutes the transition between haloid elements and clearly metallic ones… All this provides an opportunity to distribute the elements between the groups of alkali metals and haloids” (Mendeleev, 1871 [1870 on the cover]. P. 2. Issue 3).
These words illustrate how Mendeleev was creating the “poles” of the future system and what he intended to fill the interim space with. His plan was truly remarkable yet difficult to implement as he encountered the following complications:
—Not all the elements had been known by that time.
—Correct atomic weights had been determined not for all the elements (and it was unclear which ones were correct and which were not).
—The number of elements in different type (1) fragments proved to differ: five elements fitted in between Li and F as well as between Na and Cl while no less than 12 currently known elements had to be placed between K and Br:
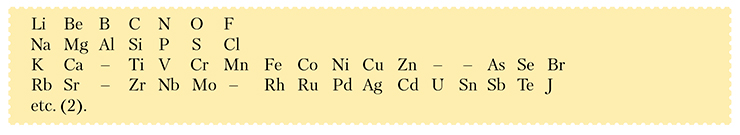
—The (Li—F) and (Na—Cl) fragments stood out from the rest not only in the number of the elements but, more importantly, in the nature of the “filling” and in the pace and rhythm of the change in the properties of the primary bodies and the corresponding compounds in the transition from an alkali metal to a halogen (for instance, the K – Br series proved to include elements whose chemistry differed noticeably from that of their direct analogues).
—Two types of analogies existed between the elements, and he had to somehow convey this fact in the system. This last difficulty deserves more detailed attention.
In version (2), the first two rows contain analogous elements standing one under another, which is natural. But look at the third row: As, a direct analogue of P; Se, a direct analogue of S; and Br, a direct analogue of Cl, they all stand aloof, sidelined by other elements. So, Mendeleev decided to break the long rows:
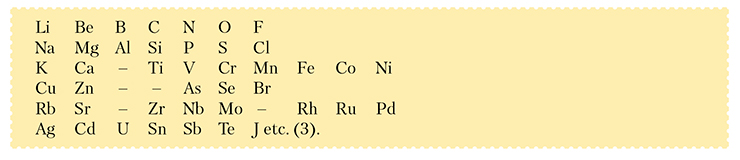
And what good came of it? Nothing. Not only some of the elements (say, Fe, Co, or Ni) came out hovering; it was all even worse. Although arsenic appeared in the same column as phosphorus and selenium in the same column as sulfur, there also were alien elements that had wormed their way between the direct analogues: vanadium wieseled its way between phosphorus and arsenic, and chromium emerged between sulfur and selenium… In what way is vanadium similar to sulfur? At first glance, in no way. But only at first glance. And Mendeleev knew it.
* Henry Enfield Roscoe, a British chemist, was one of the first to study the chemistry of vanadium. – I. D.He knew that “according to Roscoe’s* research, vanadium belongs in the nitrogen row; its atomic weight (51) compels one to place it [in the same column] between phosphorus and arsenic. The physical properties lead to the same determination of a place for vanadium; thus, vanadium oxychloride VOCl3 is a liquid having an atomic weight of 1.841 at a temperature of 14° and boiling at 127°, which is what brings it closer or, namely, places it above the corresponding phosphorus compound (i. e., POCl3 – I.D.) ” (Mendeleev, 1869, pp. 24—26).
It turns out that at a second glance, vanadium and phosphorus (as well as chromium and sulfur or chlorine and manganese) are not so alien to each other. A certain similarity exists between them, but it manifests itself in higher compounds only. For example, the highest oxidation state of chlorine and manganese is 7 (these elements would later find their place in the seventh group), and the corresponding higher compounds of these elements (Cl2O7 and Mn2O7; KClO4 and KMnO4, etc.) demonstrate similar properties. Mendeleev knew that even before 1869. Moreover, this fact had been known to many chemists before him, but the question remained: Is the similarity of the higher compounds (e. g., oxygen ones) due to the similarity between the elements themselves, which have found themselves in a special, limit state? Or maybe they have so much oxygen that it erases the differences in the very nature of the elements? This was one of the most difficult questions for Mendeleev. It took him a year, or even longer, to find an answer.
Thus, the type (3) version of the system, which works fine for us, was totally unacceptable for Mendeleev at the beginning of 1869. The main reason why he declined this version was the lack of clear-cut, strict criteria for arranging in columns the elements—as they said back then—of different razryads (‘categories’), or in the modern terminology, the subgroup A and B elements. Note that Mendeleev understood that the elements’ properties are defined not only by the atomic size and weight but also by the “inner differences in the matter of which the atoms are composed,” i. e., by the intraatomic structure (Mendeleev, 1871 [1870 on the cover]. P. 2. Issue 3). Back then, however, this insight was only a brilliant guess.

What to do next? In the situation when the criteria for joining the elements of the two razryads into one system were yet unclear, he thought it more natural to disjoin the elements of different categories. That is why, although he held in his hands a system version closely resembling by formal criteria the one that was later called the natural system, the one that today appears in school and university textbooks, Mendeleev declined to place the second-category (subgroup B) elements among those of the first category because that “would sever the naturalness of the ties between the members… of one series” (i. e., between the members of one subgroup A, as we would say today) (Mendeleev, 1869, p. 26). At first, Mendeleev even hesitated to put in one column the elements Be=9.4; Mg=24; Ca=40; Zn=65; Sn=87; Cd=112, and Ba=137.
It is only at first sight that the task of combining the elements of different categories may seem relatively easy. He had to regroup more than sixty elements, not merely discard one third of them from the system. In so doing, he had to maintain their arrangement in the increasing order of atomic weights and, possibly, the periodic of nature of change in their properties. Another complication came from the fact that Mendeleev originally considered Cu, Ag, Zn, and Cd as first-category elements (i. e., as subgroup A elements).
Perhaps, another form would do better? The one that would later be called the long form (or the long periodic table):

No, Mendeleev would not accept that arrangement either. He got perplexed over the gap in the first two rows because a vacant place within a natural system may have indicated the presence of a yet undiscovered element but he had no reasons to suspect the presence of unknown elements, say, between Be and B.
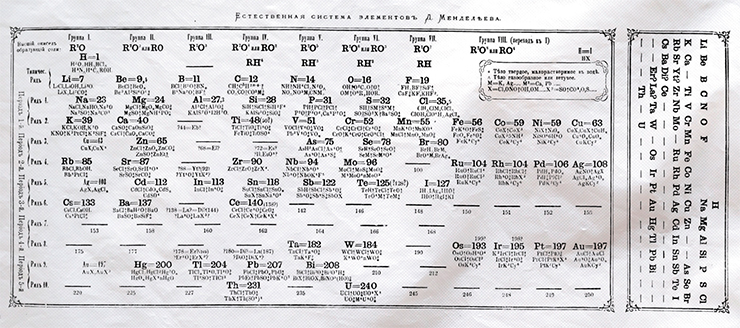
After a long struggle, Mendeleev created a version that he called, with modesty unusual for him, An Attempted System of the Elements Based on Their Atomic Weights and Chemical Analogies (in what follows, Attempted System). On the manuscript with Attempted System, he put a date February 17, 1869 (according to the Julian calendar).

The building of Attempted System and the writing of the paper “On the Correlation between the Properties of the Elements and Their Atomic Weights” drew a line under a crucial stage in Mendeleev’s work on designing a rational systematics of the elements.
At that point he was convinced that:
—Atomic weight is one of the major parameters defining the root properties of the elements; therefore, “the method of ordering elements according to their atomic weight does not contradict the natural analogies existing among the elements but, on the contrary, points directly toward them” (Mendeleev, 1869, pp. 18—20; quoted from the Engl. translation: Mendeleev on the Periodic Law: Selected Writings, 1869—1905, ed. by W. B. Jensen, 2002).
—An “accurate ratio” that exists “between the natural properties of the elements and their atomic weights” has a periodic nature in a sense that “the elements arranged by their atomic weights show a distinct periodicity of the properties.”
Experience, the son of painful errors
 Thus, Mendeleev grasped the main thing – the nature of the fundamental dependence that ties together all the chemical elements. However, the result he obtained could by no means be regarded as final because Attempted System, despite all of its merits, possessed neither integrity nor the due naturalness. For instance, transitional elements of the second category clearly demonstrated the known analogies with the first-category elements; in Attempted System, however, they served only as a canopy over the system’s framework.
Thus, Mendeleev grasped the main thing – the nature of the fundamental dependence that ties together all the chemical elements. However, the result he obtained could by no means be regarded as final because Attempted System, despite all of its merits, possessed neither integrity nor the due naturalness. For instance, transitional elements of the second category clearly demonstrated the known analogies with the first-category elements; in Attempted System, however, they served only as a canopy over the system’s framework.
Hence comes Mendeleev’s complicated attitude towards his brainchild. He included Attempted System into the first part of Principles and into the paper “On the Correlation between the Properties of the Elements and Their Atomic Weights” (not taking into account the separate sheets with the table that he printed in order to send to his colleagues), but he never published it again.
It was only in the paper “On the Place of Cerium in the System of the Elements,” which was presented to the Physics and Mathematics Department of St. Petersburg Academy of Sciences by Academician Nikolay Zinin and an adjunct A. M. Butlerov at a meeting on November 24, 1870 and published in German in the academy’s Bulletins, that Mendeleev provided a table with a simple and concise title “A System of the Elements” (Mendeleev, 1871). It was the latter that became a prototype of the currently known short form of the system, which Mendeleev called, in his other paper “A Natural System of the Chemical Elements” (1870). The visual representation of the Periodic Law in the natural system is more profound and mature. Mendeleev included it into the second part of the first edition of Principles of Chemistry (1871).
By the end of 1870, Mendeleev had realized that the “limiting” (higher) forms of oxygen compounds and their properties are determined not by the “oxygen properties themselves” and not by the presence of the “O4 edge,” i. e., an especially stable grouping of four oxygen atoms (e. g., H2SO4 ~ H2CrO4; HClO4 ~ HMnO4, etc.), but by the “state,” i. e., ultimately by the nature of the element existing in its higher oxygen compounds.
 A certain influence on Mendeleev’s speculations about the correlation between the elements of different categories could have arisen from the ideas that were put forward in 1869 by Russian chemists. Thus, Nikolay Beketov formulated, in his presentation on the atomicity (valence) concept at the Second Congress of Naturalists in Moscow in 1869, some ideas that might have attracted Mendeleev’s attention:
A certain influence on Mendeleev’s speculations about the correlation between the elements of different categories could have arisen from the ideas that were put forward in 1869 by Russian chemists. Thus, Nikolay Beketov formulated, in his presentation on the atomicity (valence) concept at the Second Congress of Naturalists in Moscow in 1869, some ideas that might have attracted Mendeleev’s attention:
“The reasons that set a limit on the bonding of two elements can be of two kinds, purely geometric and physicochemical ones. The former depend on the shape of the particles, which allows for the attachment of only a known number of particles of another body; the latter reasons, which depend on the chemical properties of matter, manifest themselves predominantly in the amount of heat released in the course of bonding. The more heat two elements can generate in the course of bonding, the more capable they are of bonding and the stronger the resulting bond. Therefore, we can imagine that the fragility of a compound that is possible by analogy will not allow it to form… So, at least two factors have an influence on the bonding limit and thus on the atomicity of the elements. Therefore it is natural that whereas one condition, which apparently remains constant (particle shape), allows for a possibility of constant atomicity, the other one, which changes (the chemical energy of the compound), affects the limit and, therefore, the atomicity itself” (Beketov, 1869, p. 236).
Another communication that might have been of interest to Mendeleev was reported at the same congress by Alexander Engelhardt. His idea was that the division of the elements into metallic and nonmetallic ones is relative; higher oxygen compounds of typical metals such as manganese and chromium have acidic properties, which is why they are closer to higher oxides of iodine, selenium, etc. Therefore, if Beketov was right, the similarity of, e. g., potassium perchlorate and permanganate as well as that of higher oxides of manganese and chlorine is due not to the influence of oxygen but to the similarity of the elements themselves, i. e., the closeness of their “chemical energies.”
“Dmitri Ivanovich, get down to work”
Let us now return to the earlier events of 1869. Mendeleev very well understood the significance of his discovery. Now he had to convince the others, to which end he first needed to introduce Russian and, importantly, foreign chemists to the discovered law and the corresponding system of the elements. This step was also important in terms of establishing his priority.
As is known, on the day he devised Attempted System, Mendeleev, who “did not get bored with studying all branches of agriculture,” was supposed to go to Tver Governorate, where he planned to inspect N. V. Vereshchagin’s artel (i. e., cooperative) cheese factories (Dmitri Mendeleev’s Archive, vol. 1, p. 58). The discovery of the Periodic Law forced him to postpone the trip by 12 days in order to finish the paper “On the Correlation between the Properties of the Elements and Their Atomic Weights.” He handed the manuscript to Nikolay Menshutkin for publication in Zhurnal Russkogo khimicheskogo obshchestva (Journal of the Russian Chemical Society (RCS))* and for presentation at the upcoming meeting of the society. Meanwhile, he went on March 1 (Julian calendar), 1869 to the cheese factories.
* Nikolay Menshutkin was a record keeper and editor of the RSC journal (Zhurn. Russ. Khim. Obshch.). In the 1860s, he became a close friend and assistant of Mendeleev.** Even today, one can find allegations in the literature that “in March 1869, at a meeting of the Russian Chemical Society…,” Mendeleev “reported on the discovery of the Periodic Law” (see, e. g., Spektor, 2017, p. 10).
Menshutkin fulfilled Mendeleev’s request. On March 6 (Julian calendar), he made on Mendeleev’s behalf a presentation on the Periodic Law.** The RSC meetings began at 8 p. m. and usually continued for two hours. Ten reports were heard on that day, mostly on organic chemistry. It is unlikely that Menshutkin had more than 10 minutes for his communication about Mendeleev’s system. The meeting minutes say: “In view of D. Mendeleev’s absence, the discussion of this communication is postponed until the next meeting” (Zhurn. Russ. Khim. Obshch., 1869, p. 35). The next meeting took place on April 3 of the same year, but the issue of a classification of the elements was raised neither at that meeting nor at subsequent ones.
The literature often raises the question: Why did not Mendeleev present his discovery himself? Different authors give different answers. In my opinion, the main reason why Mendeleev did not dare to communicate his discovery to his colleagues was that many important issues remained unresolved. Attempted System was a compromise version. In 1869, the understanding of the physicochemical part of the taxonomic problem—the periodic nature of the dependence of the elements’ properties (“chemical energy”) on their atomic weights—turned out to be much more advanced than its chemical part, i. e., the definition of criteria for putting incomplete analogues (elements of different categories) into one group.
 Perhaps, there was another reason why Mendeleev did not rush to unveil his discovery. It was absolutely clear to him that the RSC would not respond well to it because, firstly, it was a peripheral topic and, secondly, many representatives of the society had a wary attitude towards him. His student and master degree dissertations were nonexperimental works with unclear results; in Germany, studies on capillarity were treated as physics rather than chemistry; his doctoral dissertation (“On Compounds of Alcohol with Water”) was obviously a piece of applied research…
Perhaps, there was another reason why Mendeleev did not rush to unveil his discovery. It was absolutely clear to him that the RSC would not respond well to it because, firstly, it was a peripheral topic and, secondly, many representatives of the society had a wary attitude towards him. His student and master degree dissertations were nonexperimental works with unclear results; in Germany, studies on capillarity were treated as physics rather than chemistry; his doctoral dissertation (“On Compounds of Alcohol with Water”) was obviously a piece of applied research…
Academician Zinin conveyed this attitude with aphoristic brevity, “Dmitri Ivanovich, it’s time that you get down to work.” However, Mendeleev could not ignore the RSC either because it was the only professional chemical community in Russia that united chemists working in different fields. The RSC journal was the best place for a Russian publication about the discovery of the law, and such a publication required at least a formal preliminary presentation at an RSC meeting.
Thus, Mendeleev found an optimal way to publicize his work, i. e., through a communication by Menshutkin, the editor of the journal, on behalf of the author of the forthcoming publication, thus avoiding the risk of excessive polemics. It is only in sci-pop literature of a shallow and vulgar kind that one may find claims that the speech about the discovery of the Periodic Law made a colossal impression on the RSC members.
The issue of priority
When Mendeleev came back from his trip, he likely asked Menshutkin about the meeting, who said that there had been, in fact, no response and the RSC had decided to return to this issue in April.
 Meanwhile, Mendeleev himself decided—primarily from considerations of priority—to print about one and a half hundred sheets with Attempted System and disseminate them among Russian and foreign chemists. According to P. A. Druzhinin (2019), the sheets were printed around March 17: 100 copies with a Russian title and 50 with a French one. Michael Gordin believes the fact that there were twice as many Russian copies as French one means that Mendeleev targeted Russian, rather than international, audience (Gordin, 2004). In my opinion, he did so because, firstly, Mendeleev had more personal acquaintances among chemists in Russia than abroad plus he had students who were interested in novel scientific ideas and, secondly, he did it from considerations of economy (see below).
Meanwhile, Mendeleev himself decided—primarily from considerations of priority—to print about one and a half hundred sheets with Attempted System and disseminate them among Russian and foreign chemists. According to P. A. Druzhinin (2019), the sheets were printed around March 17: 100 copies with a Russian title and 50 with a French one. Michael Gordin believes the fact that there were twice as many Russian copies as French one means that Mendeleev targeted Russian, rather than international, audience (Gordin, 2004). In my opinion, he did so because, firstly, Mendeleev had more personal acquaintances among chemists in Russia than abroad plus he had students who were interested in novel scientific ideas and, secondly, he did it from considerations of economy (see below).
Note that sheets with Attempted System that were printed in March 1869 contained no explanations to the table. This was done for a reason—Mendeleev was in a haste to establish his priority. He had no competitors in Russia, but many scientists abroad attempted to classify the elements and, so to say, were snapping at his heels. If he had included additional information into the sheets, he would have needed to obtain an official permission to print them, and that would have taken time (Druzhinin, 2019). Meanwhile, before his paper came from the press, he needed to make a step, however small, to establish his priority.
Note that Mendeleev wrote on the final draft of Attempted System, “Take paper that can be written on yet thin enough to keep it light [in terms of weight].” Druzhinin comments on this, “light paper was chosen for a reason—Mendeleev, a person who knew to spend money wisely, wanted the letter not to exceed the minimum weight for overseas mail (15 g with the envelope and, possible, an accompanying note) because the lowest price for the delivery of one such letter to the states of the German Postal Union was 14 silver kopecks” (Ibid., p. 118). As you can see, Mendeleev was reluctant to pay out of his pocket even the additional expenses on the establishment of the Periodic Law.
As early as in April 1869, the French version of Attempted System was published in the German Journal für praktische Chemie (Mendeleeff, 1869).
Of course, Mendeleev understood that the dissemination of the copies of Attempted System would not be enough to guarantee his priority. Therefore, as soon as the RCS journal with his paper on the Periodic Law* saw the light, he immediately, in May 1869, prepared its short abstract in three parts: the title, the table (Attempted System), and the main conclusions. Mendeleev intended to publish this abstract in Zeitschrift für Chemie, a monthly German semiabstract journal, which was founded by a group of professors of Heidelberg University.
* No later than on May 8 (Julian calendar), 1869 (Druzhinin, 2019).Mendeleev, who learned the German language at the gymnasium and at the institute and then spent two years in Germany as an intern, nevertheless, did not feel himself confident in his language skills, especially when it came to writing a scholarly paper. Therefore, he availed himself to the offer of one of the journal editors, Friedrich Beilstein, to submit the papers and abstracts in Russian only.
However, Beilstein, who himself was head over heels with work, handed the translation of the abstract over to A. Ferman, his assistant at the Institute of Technology. When translating the first and most important statement in the abstract—“the elements arranged according to their atomic weights demonstrate an apparent periodicity of the properties,” Ferman used, instead of periodicity, the term stufenweise, meaning a stepwise change (Mendeleeff, 1869, p. 405).
 To that Bonifati Kedrov (1953) stated categorically, out of the habit typical of many Soviet and Russian authors to see vicious intrigues in any mistake or stupidity, “These distortions cannot be accidental; they testify to the malicious intent of the translator, who perverted the core idea of the great discovery made by the Russian scientist, thus trying to present it as a simple tabulation of the elements.” It never occurred to Kedrov that in order to consciously pervert the “core idea of the great discovery,” one had to first comprehend it and appreciate its greatness. But this we see neither in 1869 nor later, in any of Mendeleev’s contemporaries, even those who concerned themselves with classification of the chemical elements. As to the presentation of the great discovery of the Russian scientist “as a simple tabulation of the elements,” what would Kedrov say, had he lived to the present day and found out that by the initiative of the Russian Academy of Sciences and the Mendeleev Russian Chemical Society, the year 2019 was declared as the International Year of the Periodic Table?
To that Bonifati Kedrov (1953) stated categorically, out of the habit typical of many Soviet and Russian authors to see vicious intrigues in any mistake or stupidity, “These distortions cannot be accidental; they testify to the malicious intent of the translator, who perverted the core idea of the great discovery made by the Russian scientist, thus trying to present it as a simple tabulation of the elements.” It never occurred to Kedrov that in order to consciously pervert the “core idea of the great discovery,” one had to first comprehend it and appreciate its greatness. But this we see neither in 1869 nor later, in any of Mendeleev’s contemporaries, even those who concerned themselves with classification of the chemical elements. As to the presentation of the great discovery of the Russian scientist “as a simple tabulation of the elements,” what would Kedrov say, had he lived to the present day and found out that by the initiative of the Russian Academy of Sciences and the Mendeleev Russian Chemical Society, the year 2019 was declared as the International Year of the Periodic Table?
It is likely that the term periodic—now I return to the 19th century—appeared inadequate to the translator because in mathematics, a periodic function is a function whose values recur after a certain regular interval of the argument. The system of the elements is, strictly speaking, not such a case—it is the analogues that recur after a certain number of the elements (which is different in different cases). In other words, the strict mathematical understanding of a periodic change becomes fuzzier in the system of the elements. Meanwhile, the German noun Stufe means a step, staircase, stage, tier, phase, interval, gradation. It seems that the translator decided that Mendeleev meant an alternation of the elements’ properties that resembles that of staircases or theatrical tiers. It would be incorrect to say that this understanding, despite all its drawbacks, completely perverted the core idea of Mendeleev’s discovery.
Chemists and chemistry teachers, especially in Russia, have traditionally drawn a distinction between three concepts: the Periodic Law, the Periodic System, and the Periodic Table (sometimes the last two are identified with each other). The Periodic Law is the law that underlies the systematics of the elements and (in Mendeleev’s wording) states: “The physical and chemical properties of the elements, manifested in the properties of simple and complex bodies that they form, are periodically dependent… on their atomic weight.” Currently, this law has a different formulation (the properties of the elements are put in a periodic dependence on the charge of the nucleus); nevertheless, it remains a major law of nature.
The Periodic System defines the general principles underlying the systematization of the elements in accordance with the Periodic Law, i. e., the topology of interelement relations.

The Periodic Table is a visual representation of the Periodic Law and System. As Mendeleev put it, “there could be more of such distributions. They do not change the essence of the system” and, I would add, the essence of the law. Today, several hundred variants exist of the visual representation of the Periodic System (Mazurs, 1974).
Unfortunately, one is now beginning to forget these basic notions. This tendency manifested itself, e. g., in the fact that through the efforts of the Russian scientific elite, the year 2019 was declared the International Year of the Periodic Table, not the Periodic Law, which is illiterate from both the historical and chemical point of view but goes well with the image-oriented and shallow character of the modern Russian culture.
References
Arkhiv D. I. Mendeleeva. Tom 1. Avtobiograficheskie materialy. Sbornik dokumentov (Dmitri Mendeleev’s Archive. Vol. 1. Autobiographic Materials. Collection of Documents) / Comp. by M. D. Mendeleeva and T. S. Kudryavtseva. Ed. by S. A. Shchukarev and S. N. Valk. Leningrad, 1951. P. 52 [in Russian].
Druzhinin P. A. Zagadka “Tablitsy Mendeleeva”: Istoriya publikatsii otkrytiya D. I. Mendeleevym Periodicheskogo zakona (The Mystery of Mendeleev’s Table: A History of the Publication of Dmitri Mendeleev’s Discovery of the Periodic Law). Moscow: Novoe Literaturnoe Obozrenie, 2019. (Ser. Istor. Nauki). P. 118 [in Russian].
Gordin M. D. A Well-Ordered Thing. Dmitrii Mendeleev and the Shadow of the Periodic Law. New York: Basic Book, 2004. P. 28.
Mendeleev D. I. On the Correlation between the Properties of the Elements and Their Atomic Weights // Mendeleev D. I. Periodicheskii zakon. Osnovnye stat’i (The Periodic Law: Main Papers), Ed. by B. M. Kedrov. Moscow, 1958 [in Russian].






