Variola Virus: Evil for Good
Both humans and animals have always had to struggle with pathogenic microorganisms, which has led to the development of innate and adaptive immune systems. These systems protect us from external “aggressors”; however, their operation may be disturbed, which leads to pathological states accompanied by chronic, hard-to-cure inflammatory and autoimmune responses. The therapies of asthma, atherosclerosis, systemic lupus erythematosus, psoriasis, rheumatoid arthritis, multiple sclerosis, etc. use human antibodies, which deactivate proteins involved in immune responses. However, the proteins of pathogenic microorganisms, e.g., viruses, which are evolutionarily adapted to overcoming the body’s defense systems, can also be used for this purpose. Of special interest to medical biotechnology are highly pathogenic viruses for which the man is the only host. Today, the proteins of the variola virus, which had been a scourge of civilizations for centuries, are used for making drugs to prevent the most severe inflammatory and autoimmune diseases
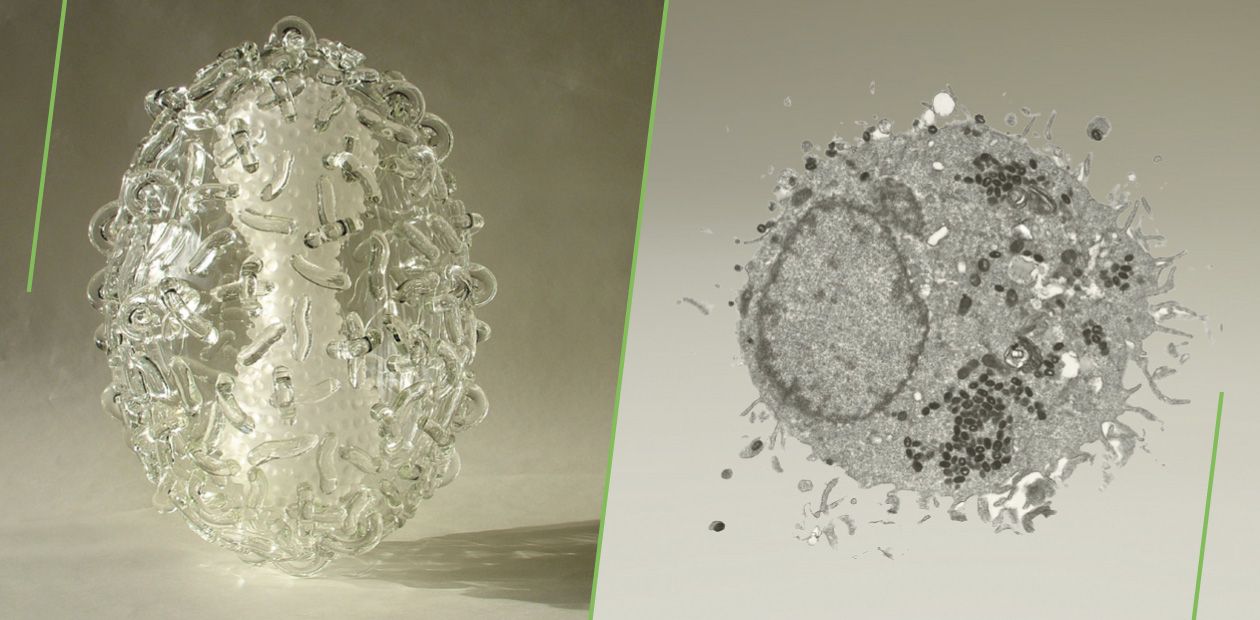
Right up to the discoveries by Antoni van Leeuwenhoek in the 17th century, the humankind had been unaware of the fact that it lived in the ambience filled with multitudes of various most minute creatures. These microorganisms are so small that one can see them only with the help of a good microscope, an optical one for studying bacteria and protists, or a complex and expensive electron microscope for obtaining the “portraits” of viruses.
Most of the microorganisms coexist with humans and animals peacefully, and many of them do it in a mutually beneficial way; however, some microbes break the “peace treaty” and become pathogenic. Amazing though it may seem, the ability of such pathogenic microorganisms to overcome the defense systems of host organisms can be used for our good. Of special interest for the current medical biotechnology are specific pathogens, such as variola virus, for which a human is the only host. Proteins of such viruses are now used to design the drugs able to treat severe chronic inflammatory diseases of noninfectious nature, including autoimmune disease
Microorganisms live everywhere, be it air, water, soil, or our own bodies… As compared with humans and animals, they reproduce very rapidly, and their communities are immeasurably more numerous. In particular, considering the humankind on a microcosmic scale, a standard microbiological flask would easily lodge all the seven billion people.
In order to survive, both humans and animals had to constantly struggle with the surrounding pathogenic micro-organisms. Such events in the evolutionary history have determined the emergence and establishment of numerous defense systems in mammalian organisms, which provide for their survival in the “microbial broth” of the thin biosphere layer of our planet.
The most “immediate” response among the body’s defense systems is a nonspecific response, i.e., the response targeted against any viruses, microbes, and biological macro¬molecules. This refers to the innate immunity tuned to recognition of and further response to molecular components of the microorganisms that pose a threat to the organism. The well-known inflammatory processes, preventing the spreading of a pathogen in the first hours and days after being infected, are important players in the nonspecific defense of the body against diseases.
IMMUNE DEFENSE The goal of the immune system is to protect the body from internal and external threats.The innate immunity is nonspecific; it provides the defense against any infectious agents, be they viruses or bacteria, as well as macromolecules.
Apoptosis, a programmed cell suicide, is one of the first (probably, also one of the most ancient) lines in this nonspecific defense. In the case of a virus infection, apoptosis, induced by molecular recognition of specific molecules of a pathogen, prevents virus reproduction and infection of other cells in the host body.
Inflammation, developing in the first hours and days after infection and focused on virus confinement, plays an important role in the early nonspecific defense against virus infection. The cells constituting the innate immune system in mammals–macrophages, dendritic cells and natural killers–respond to infection by producing the so-called proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-18 (IL-18), tumor necrosis factor (TNF), and γ-interferon (IFNγ). Proteins belonging to another class, chemokines, which regulate the leukocyte transport and function, are also involved in development of inflammatory response.
Complement, a multicomponent set of blood serum complex proteins, is an additional mechanism of the innate immunity; these proteins are proteolytic enzymes involved in inactivation of infectious agents and infected cells.
The body’s specific defense targeted to a particular infectious agent–adaptive or acquired immunity–develops slower as compared with the innate immune response. It involves an intricate interaction between various types of cells under the control of the cytokines TNF, IL-1β and IFNγ. The result of this response is appearance of B lymphocytes, which produce specific antiviral antibodies, and virus-specific cytolytic T lymphocytes. Specific antibodies interact with virus particles and their components either in an individual manner or in complex with the complement and inactivate them.
Thus, IL-1β, TNF, and IFNγ are the most important cytokines, which, along with regulation of inflammatory response, control development of the adaptive immune response to infection
An organism’s specific defense against an individual infectious agent—an adaptive or acquired immunity—develops slower; it is an intricate interaction of various immune cells regulated by specific proteins.
All these systems protect us from external “aggressors”; however, operation of these systems may be disturbed, which leads to pathological states accompanied by chronic inflammatory and (or) autoimmune responses, such as asthma, atherosclerosis, systemic lupus erythematosus, psoriasis, rheumatoid arthritis, multiple sclerosis, and other severe untreatable diseases.
Currently, the so-called “biological” therapies of such diseases are being developed, which are based on blocking excess activation of defense systems with the help of various biological macromolecules. One of the methods is to use human antibodies, which can specifically interact with and deactivate the proteins involved in innate and adaptive immune responses, overproduction of which leads to pathologies.
And what if we use for this purpose some proteins of pathogenic microorganisms, such as viruses, which are evolutionarily adapted to overcoming a body’s defense systems, thus making lemonade out of lemons?
Pathogenic and anthroponotic
The kingdom of viruses was discovered over a hundred years ago by D. I. Ivanovsky, an outstanding Russian scientist; however, only in the last decades the rapid development of instrumental research methods has made it possible to gain an insight into these minutest organisms.
Although viruses are most diverse in their structure and function, they all are able to reproduce only in the cells of other organisms, be they unicellular or multicellular. In the long-term coevolution with their hosts, viruses constantly “test” new ways of suppressing the host defense responses or “escaping” these responses by using molecular mimicry. For example, viruses can include into their genomes the coding sequences of cellular genes involved in regulating the immune response and modify them, adapting them to their own life activity.
Various mammalian viruses differ not only in the size of their genomes and particles, but also in the strategies of their development in the host organism. Viruses belonging to different families demonstrate an extraordinary diversity of mechanisms used to overcome the innate and adaptive immunities of mammals. Consequently, research into the specific features of viruses makes it possible to find new patterns of organization and functioning of animal and human defense systems, which are responsible for the recovery of infected organism.
Most viruses are able to infect a wide range of animal species (host range). However, of special interest to medical biotechnology are highly pathogenic and anthroponotic viruses, with a human as the only host. In this case, the virus can very efficiently suppress (or escape) the immune responses of a human organism. Genotypic studies of such viruses and detection of the viral proteins that efficiently inhibit development of inflammatory response to infection form the background for designing new drugs that will be able to treat chronic inflammatory diseases of noninfectious nature.
The variola (smallpox) virus is an amazing example of both high pathogenicity for humans and of strict anthroponotic nature. As is postulated, this virus initially had a wide host range but during its evolution it lost the ability to reproduce in other mammals; it was,preserved in an endemic (that is, characteristic of only particular area) state for many centuries in densely populated regions, first and foremost, of the Indian subcontinent (Shchelkunov, 2012).
Note that the variola virus cannot stay in the human body in a latent state or cause a chronic infection: the disease always ends in either recovery of an infected person or death. This decreases the virus’s ability to survive in wildlife and suggests that it has come to a certain evolutionary “dead end”. On the other hand, reproducing exclusively in the human body for many generations, the variola virus has maximally adapted, on a molecular level, to overcoming the multilevel mechanisms of human innate and adaptive immunities (Shchelkunov, 2011).
These properties of a special pathogen, variola virus, suggest that its proteins may be useful for the therapy of various human immunopathologies (Shchelkunov, 1995). Currently, the State Research Center of Virology and Biotechnology Vector (Koltsovo, Novosibirsk oblast, Russia) is involved in elaborating of new generation drugs based on the proteins secreted by poxviruses, including the variola virus.
Researchers of the State Research Center of Virology and Biotechnology Vector (Koltsovo, Novosibirsk oblast, Russia) were the first to decode the genome structures of variola, cowpox, and monkeypox viruses, pathogenic for humans, isolated from infected persons (Shchelkunov, 1993, 1998, 2001). Comparison of the genomic strategies of these viruses and their immunomodulatory proteins has demonstrated that characteristic of all viruses belonging to the family Poxviridae is the highest diversity of the mechanisms underlying their ability to overcome human immune defense responses as compared with the viruses belonging to other families (Shchelkunov, 2012). Variola virus, the only host for which is human, is the most efficient as compared with the remaining poxviruses in inhibiting the human immune systemThe weapon against inflammation
As has been mentioned, the major cause of autoimmune diseases is a disturbance in the immune system, including overproduction of the substances that induce inflammatory responses.
One of the key cytokines involved in the inflammatory immune response is the tumor necrosis factor (TNF); the increased production of this factor causes such autoimmune diseases as psoriasis, Crohn’s disease, and rheumatoid arthritis. The high level of TNF production also determines a severe pathology frequently leading to a lethal outcome, the so-called septic or endotoxic shock.
It is possible to prevent TNF binding to its protein receptors, which are anchored to the cell membrane, using corresponding monoclonal antibodies or the so-called soluble forms of TNF receptors, i.e. the extracellular domains (regions) of receptor proteins, commonly found in serum and other biological fluids. Binding of such molecules to TNF should inactivate this cytokine and reduce the inflammatory process it induces.
Indeed, the therapeutic effect of anti-TNF antibodies has been demonstrated in model laboratory experiments when treating several pathologies caused by an increased TNF production. However, the attempts of a direct use of soluble cellular TNF receptors have failed so far. On the other hand, genetically engineered chimeric proteins comprising the TNF-binding part of cellular receptors and a fragment of human immunoglobulin were a success.
For several years now, a few of such biological therapeutics designed on the basis of human proteins have been successfully applied in clinical practice for treating inflammatory diseases of noninfectious nature. First and foremost, these are Etanercept (with a chimeric protein as the major component), Infliximab, and Adalimumab (the last two involve human monoclonal antibodies); two analogous drugs recently supplemented this list.
However, clinical studies have demonstrated that patients with rheumatoid arthritis or other inflammatory or autoimmune diseases are selectively sensitive to only one of these anti-TNF drugs. In addition, all these drugs are of protein nature, and thus, they themselves are target for the immune system of patients, which suggests a decrease in their efficiency with time in the case of a long-term therapy. This means that once a patient becomes insensitive to the administered drug, it should be replaced with another one.
RHEUMATOID ARTHRITIS is a systemic autoimmune disease of the connective tissue appearing as a chronic inflammation of joints (ankles, knees, and hands).Currently, each hundredth of the world population suffers from this disease, constituting over 70 million people. Women are affected severalfold more frequently as compared with men. Typically, the disease develops after the age of 30 and leads to an early disability onset in 70% of the affected people.
The causes underlying this disease are rather vague; however, it is known that a failure in the immune system caused by overcooling, stresses, injuries of joints, and infection, including acute respiratory diseases, tonsillitis, and influenza is a possible reason. The proinflammatory cytokines, such as tumor necrosis factor and γ-interferon, are overproduced in rheumatoid arthritis
Now, new medications involving viral proteins may be of great help to therapists. For example, some anti-TNF drugs based on poxvirus TNF-binding proteins are being developed at the State Research Center of Virology and Biotechnology Vector.
The genetic engineering approach has allowed Vector’s researchers to construct recombinant baculoviruses that produce in cell culture a TNF-binding protein (CrmB) characteristic of cowpox, monkeypox, and variola viruses. However, tests in an experimental model of endotoxic shock have shown that only the variola virus protein CrmB displays a pronounced therapeutic effect (Gileva et al., 2006). Either this protein or its reconstructed variants may become an effective agent for new anti-TNF therapeutics (Gileva et al., 2009).
SEPSIS (the Greek word for putrefaction and decay) is a severe human infectious disease developing as a systemic inflammatory response to infectious agents (bacteria or unicellular fungi) or their toxins that entered the blood. Severity of this disease is frequently associated with development of the so-called septic shock (infectious toxic or endotoxic shock), which is more typical of infection with gram-negative bacteria and staphylococci and is accompanied by lung, liver, and kidney dysfunctions and changes in the blood clotting system.Despite considerably grown capacities of the state-of-the-art antibacterial and antifungal therapies, the mortality rate of sepsis still reaches 25–30%. Currently, this disease is still among the leading mortality factors: in the United States, it is guilty of over 200 thousand deaths each year.
It has been proved that an infection itself is not the direct cause of numerous pathological changes characteristic of sepsis. They rather appear as a result of the organism’s response to infection and several other factors determined by the effect of various endogenous regulatory substances. Such molecular responses may be regarded as an adaptation in a normal state; however, their overactivation in the case of sepsis is harmful.
Overproduced tumor necrosis factor, γ-interferon, and several interleukins are the major players as mediators in septic shock
The “Klondike” of pharmaceutics
The medical potential of viral proteins is far from being confined to anti-TNF therapy. In particular, the well-known gamma-interferon (IFNγ) is another inflammatory cytokine. Currently, the drug Fontolizumab, designed on the basis of human monoclonal anti-IFNγ antibodies and intended for treatment of several autoimmune diseases, is in the second stage of clinical trials.
The IFNγ-binding protein of the variola virus may also emerge to be an efficient IFNγ inhibitor. Such a protein, produced at the Vector, not only efficiently inhibited the protective effect of human IFNγ in the human fetal lung cell culture infected with the mouse encephalomyocarditis virus, but also exceeded in efficiency the drug based on human cellular IFNγ receptors (Nepomnyashchikh et al., 2005).
There are numerous data confirming that the inflammatory process and histopathological alterations in many inflammatory and autoimmune diseases of the nervous system, in arthritis, glomerulonephritis, systemic lupus erythematosus, and in other diseases are, in many cases, determined by activation of the blood complement system, another system of nonspecific immunity. The complement also plays an important role in graft rejection.
Poxviruses encode a special protein (CBP) capable of inhibiting activation of the complement; again, it is the variola virus protein that interacts most efficiently with the human complement proteins. This particular protein is now regarded to be a promising drug for treating Alzheimer’s disease, multiorgan dysfunction syndrome, and xenograft rejection (Jha and Kotwal, 2003). Thus, experiments with laboratory animals have demonstrated that a recombinant CBP enhances restoration of the brain function after moderate and severe craniocerebral injuries. This protein also appears to be effective for treating spinal cord injuries: its administering considerably reduces the histopathological alterations caused by inflammatory response.
Chemokines, a large family of small proteins with very similar tertiary structures, also play an important part in the pathogenesis of inflammatory and autoimmune diseases. Poxviruses code for chemokine-binding proteins, which have no homologs in their amino acid sequences among the known vertebrate proteins. The high therapeutic potential of chemokine-binding proteins has been shown using several laboratory models of inflammatory and autoimmune diseases (Nepomnyashchikh and Shchelkunov, 2008).
Thus, the hypothesis postulating that the viral proteins acting as antagonists of immune regulatory proteins have a therapeutic potential has been reliably confirmed. Currently, the new generation drugs for treating the human patho-logies caused by overactivation of mediator proteins involved in the body’s defense systems are being constructed by genetic engineering methods.
This standpoint allows for a fresh look at the variola virus: the results of laboratory studies and preclinical trials demonstrate a high potential of the drugs that are based on proteins of the variola virus, which was a scourge of civilizations for centuries; these drugs will be able to prevent the most severe inflammatory and autoimmune diseases.
References
Nepomnyashchikh T. S., Shhelkunov S. N. Poxviral immunomodulating proteins: New tools for immunity correction // Molecular Biology. 2008. V. 42, N. 5. P. 806—813.
Shhelkunov S. N. Evasion of mammalian defense systems by orthopoxviruses // Molecular Biology. 2011. V. 45, N. 1. P. 24—35.
Shchelkunov S. N. Orthopoxvirus genes that mediate disease virulence and host tropism // Advances in Virology. 2012. Vol. 2012, Article ID 524743, 17 p. DOI:10.1155/2012/524743.
Shhelkunov S. N. Virus natural’noj ospy – istochnik novyh medicinskih preparatov // Sorosovskij obrazovatel’nyj zhurnal. 1995. № 1. S. 28—31.
Shhelkunov S. N. Geneticheskaja inzhenerija: Ucheb.-sprav. posobie. 3-e izd., ispr. i dop. Novosibirsk: Sib. univer. izd-vo, 2008. 514 s.
Shhelkunov S. N. Ospa – damoklov mech civilizacij // Nauka iz pervyh ruk. 2012. № 6 (48). S. 96—109.
The author and Editorial Board thank D.V. Antonets (State Research Center of Virology and Biotechnology Vector) for assistance in artwork
The work was supported by the Russian Foundation for Basic Research (grant No. 12-04-0011a)