Halt, Encephalitis Fires!
The thermometer stem outside the window paused near the zero mark, and the first snow concealed the gold of Indian summer… At last the reports of the Department of Epidemiological Surveillance, most similar to war briefs, disappeared from the Novosibirsk City Site. The summer is over together with the “tick season”. It’s high time to recon up the casualties: over 19 thousand people bitten by ticks went to clinics of our city and oblast; of them, 505 were hospitalized; the diagnosis of tick-borne encephalitis was confirmed for 116 cases, and 3 people out of them died.
In the list of the vectors of human communicable diseases, ticks occupy the second position after mosquitoes: at least 3 viral, 22 bacterial, and several protozoan infections transmitted by ixodid ticks have been detected so far. The situation is often complicated by the fact that biting ticks are capable of transmitting to humans concurrently various species of bacteria, viruses and protozoan, thereby causing mixed infections, which often have a more severe course. Potential circulation of the pathogens in agricultural animals and pets (for example, infectious agents can remain in dairy products), as well as blood transfusion and organ transplantations bring about additional risks of infection.
The most dangerous of the tick-borne infections are tick-borne encephalitis, ixodid tick-borne borreliosis, ehrlichiosis, rickettsiosis, and babesiosis. The most socially important pathogens for Russia are borrelia and, of course, tick-borne encephalitis virus, which is portrayed below.
Taiga Infection
The Far East was intensively developing in the 1930s: roads were built, forests were cut down, and large military units were stationed in taiga due to the tension with Japan. The physicians that worked at that time in Primorsky krai commenced regularly reporting an unknown severe disease affecting both the aboriginal population and the military. The disease, regarded as a new variant of severe influenza, was accompanied by a sudden fever and frequently led to paralyses and even death.
Only in 1935, A. G. Panov, a local physician, succeeded in diagnosing correctly this unknown disease. It appeared to be a brain inflammation, i.e., encephalitis, similar in its symptoms to the previously described Japanese encephalitis. In 1935, physicians of the Far Eastern Pasteur Station attempted to isolate the agent of this disease by injecting the brain emulsion of human lethal cases into the mouse brain. Although the mice developed the signs of disease, this study was unsuccessful.
In January 1937, military physicians addressed the USSR Narkomzdrav (People’s Commissariat of Public Health), and this agency decided to organize a scientific expedition to the Far East, with L. A. Zilber as its head. A real virological laboratory was deployed under the hard field conditions. The scientists fulfilled their task: they succeeded in isolating and describing the pathogen that caused severe illnesses of the central nervous system. In addition, the determinative role of ixodid ticks in transmission of this infectious agent was distinctly demonstrated. It appeared that wild vertebrates were the source of tick infection, and this formed the basis for developing recommendations on the necessary prevention measures.
Unfortunately, this truly brilliant discovery, an important milestone in the history of virology, took its victims among the members of the scientific expedition. Among them were M. P. Chumakov, a future academician and founder of the Institute of Poliomyelitis, who contracted a severest illness that passed into a chronic form lasting to the end of his days; and V. D. Solov’ev, who went blind for six months as a sequela of the tick infection.
A Dangerous Neighbor
What is known today about tick-borne encephalitis virus (TBEV)? The virus belongs to a considerably old, from the evolutionary standpoint, family of flaviviruses (Flaviviridae), comprising over 70 animal and human viruses including very dangerous pathogens, such as yellow fever, Japanese encephalitis, Dengue fever, and hepatitis C viruses.
Lev A. ZILBER (1894—1966) is one of the founders of medical science in the USSR. His name is associated with fundamental research into variation of bacteria and the nature of immunity; organization of the first in this country virological centers; creation and experimental development of the virus-genetic theory of tumor etiology; and development of cancer immunology, a novel line of investigation.In 1937 Zilber was awarded the Prize of the USSR Narkomzdrav for detection of the agent and transmitter of spring–summer encephalitis. He was arrested in the same year on the false accusation that the expedition he headed had been secretly spreading the Japanese encephalitis in the Far East. Zilber was released one and a half years later. During the short period between his release and a new arrest, Zilber prepared the monograph about epidemic encephalitides and several scientific papers.
In 1940, Zilber was arrested again, accused of treason, espionage, and sabotage, and convicted for 10 years. He was released in 1944 thanks to the plea of his friends and prominent scientists and was elected a member of the USSR Academy of Medical Sciences in 1945. Although Zilber was mostly in confinement while the war was on, after the war he was awarded the Medal for Valiant Labor during the Great Patriotic War of 1941—1945. Actually, Zilber deserved this medal, as in the labor camp he had saved thousands of lives by healing, releasing people from hard labor, and just by words of sympathy and support.
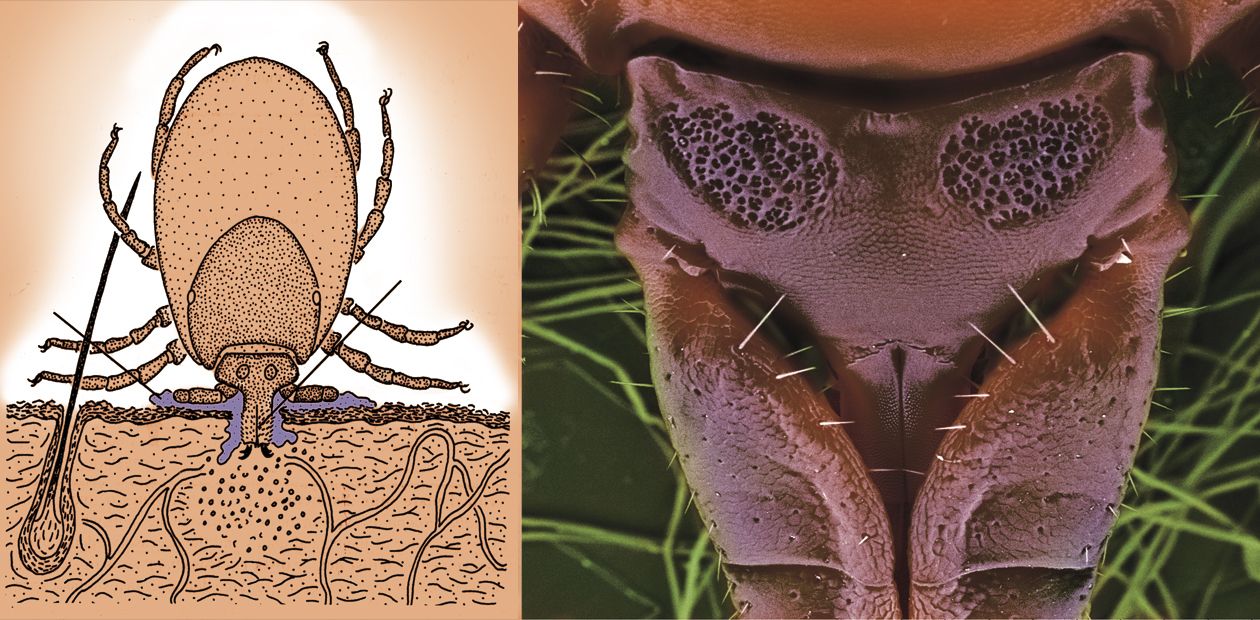
The main natural reservoir of TBEV is small mammals (voles, mice, and insectivores). The virus is able to infect animals and reproduce in their bodies; however, the disease does not often do any noticeable harm to the animals’ health. The virus vectors are ticks, which feed on the blood of forest animals — the European castor bean tick, taiga tick, marsh tick, and several other rarer species. TBEV can propagate in their bodies too. It is not known precisely whether the virus was initially connected with ticks alone or with vertebrates, but it has adapted to live in both organisms during the evolution.
Nowadays tick-borne encephalitis virus is found in the forest areas all over the territory of Eurasia from the Atlantic to the Pacific ocean; in addition, the area it inhabits coincides on the whole with the areas of the European castor bean and taiga ticks. During the last decades, the area of TBEV has steadily expanded due to the intensifying human activities. For example, small bushes grow over the abandoned glades and assist their turning into swamps, thereby providing ideal conditions for habitation of small animals and the ticks connected with them.
In addition, an ever increasing number of people prefer to spend their free time in nature’s lap, resting or working on their land plots. For example, about 75 % of the population of the Novosibirsk oblast lives in the areas favorable for ticks; the majority of recreation facilities and areas, summer houses, and small summer farms are concentrated there too.
Researchers from the Institute of Chemical Biology and Fundamental Medicine studied 95 strains of tick-borne encephalitis virus from the collection of the Institute of Animal Systematics and Ecology, Siberian Branch of the Russian Academy of Sciences. The strains were isolated from the adult taiga ticks collected in 1980—2001 from the vegetation in the eastern part of the Novosibirsk oblast.Analysis of the nucleotide sequences of E gene, encoding virus envelope protein, has demonstrated that all the strains belong to Siberian genotype.
On the other hand, collaborative study with Novosibirsk physicians has detected the TBEV isolates of Far Eastern genotype in the blood of patients hospitalized with suspected tick-borne infections.
It was believed earlier that the Far Eastern type caused only a severe form of the illness, whereas the Siberian type was responsible predominantly for chronic diseases. Our studies, confirmed by the data of other researchers, have demonstrated that the virus of Far Eastern genotype is able to cause various tick-borne encephalitis variants, from the most severe to inexplicit, without any manifestations.
The genome of tick-borne encephalitis virus was decoded in 1989—1990 virtually simultaneously in this country (in particular, at the Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences; Pletnev et al., 1990) and abroad (Mandl et al., 1989). So far three genetic types of this virus have been isolated, which differ in their properties, namely, Far Eastern, Siberian, and Western European genotypes. This correspondence of the genotypes with geographic locations is rather arbitrary, as the strains belonging to different types may occur in the same region.
Similarly to the majority of other viral infections, there is no highly specific therapy efficient against tick-borne encephalitis virus — therein lies its menace. The therapy for all viral diseases aims mainly at stimulation of the immunity and elimination of outer signs of the illness, which means that the organism should cope with the infection on its own. Another menace of tick-borne encephalitis is its ability to cause chronic diseases and sequelae, such as paralyses and disability. Therefore, the most important component in the research into this pathogen is the development of diagnostic and prevention tools.
Diagnostics and Prevention
Serological methods are the most widely used in the diagnostics of tick-borne encephalitis; they allow the antibodies, specific protective proteins, or the pathogen itself to be detected in the patient’s blood. However, these techniques are insufficiently sensitive and do not exclude possible false results due to cross-reactions with other pathogens. In addition, they are unable to characterize in detail the pathogen itself (for example, to determine its genotype).
Molecular genetic methods, capable of recognizing the viral genetic material in a specific manner, display a higher sensitivity. This is especially true for polymerase chain reaction** (PCR). This method makes it possible to replicate and identify a tiny amount of the virus hereditary material, thereby allowing for the diagnostics during the first days after infection. However, the merits and demerits can interchange under certain circumstances: a high sensitivity of molecular genetic techniques may also lead to errors in diagnostics of tick-borne encephalitis due to new mutations in viral genomes; on the other hand, this technique requires high purity experiments.
Immunization with corresponding vaccines is today the most important measure in prevention of tick-borne encephalitides. The most popular vaccines against the flavivirus infections are inactivated (killed) vaccines produced in cell cultures infected with the virus and then treated with specific chemical compounds. In the late 1970s, this particular method was used to produce a purified vaccine against tick-borne encephalitis, which has become widespread in the countries of Europe and Asia. The vaccine involving a chemically inactivated “relative” of TBEV, the Japanese encephalitis virus, is also used in the prevention of tick-borne encephalitis.
Such inactivated viruses and purified viral proteins are safer compared with another vaccine type, the so-called “live” vaccines, containing attenuated virus strain as the main component. However, the vaccines of this group are often more efficient, as the chemical inactivation of infectious agents can partially impair the structures of the viral proteins that are to induce the protective immunity.
Live Vaccines
Live vaccines is a way to stimulate the immunity via introducing attenuated viruses into the organism, discovered as long ago as in 1796 by the English physician E. Jenner. Since that time, a live vaccine against the yellow fever virus has been designed, which differs from highly pathogenic strains by numerous nucleotide substitutions in the viral genome; and attenuated strains of other flaviviruses — Dengue, West Nile, and Langat — have been searched for using them in designing the live vaccines.
Development of the live vaccine against tick-borne encephalitis virus was also attempted. In 1957, it was decided to use as such a live vaccine the attenuated flavivirus strain Langat, inducing the antibodies similar to the antibodies to TBEV. However, it appeared that this strain recovered pathogenicity upon an intracerebral introduction and itself caused encephalitides and atrophy of brain regions without any outer clinical manifestations. Several attenuated strains of TBEV were later found; unfortunately they all appeared genetically unstable.
There was a tragic example of administering the live vaccines against TBEV. In 1961, an attenuated strain of tick-borne encephalitis virus was isolated of a patient who displayed no clinical signs of the disease during four years after the tick bite but retained high antibody titers in the blood. The laboratory studies demonstrated a low neurovirulence of this strain, and the subsequent clinical trials carried out on volunteers gave positive results. Eventually, about 650,000 people were immunized with this attenuated TBEV strain. However, 35 vaccinees developed severe complications — meningites or meningoencephalitides; moreover, 22 of them retained severe sequelae for the rest of their lives and one patient died. The administration of this strain as a live vaccine was stopped (Timofeev, Karganova, 2003).
DNA Copies
Attenuated virus strains used to be obtained by selection following a long-term cultivation; at present, the method of the so-called site-directed mutagenesis has become widespread. For this purpose, full-size DNA copies of viral RNA genomes are produced wherto certain mutations are introduced by genetic engineering techniques. Then such a DNA serves as a template for synthesizing the corresponding viral RNA in vitro. Applying such a recombinant RNA into cells, it is possible to obtain the attenuated viruses with prespecified properties.
These manipulations made it possible to create live chimeric vaccines containing, for example, part of the genes of yellow fever virus and part of the genes of other flaviviruses. Note that the point mutations decreasing their pathogenicity were introduced into the functionally important regions of the genomes (Pletnev et al., 2006).
Nonetheless, administration of attenuated live vaccines, including the vaccines against TBEV, is limited due to the probability that attenuated strains will recover a high pathogenicity of the wild type strains. This occurs because the host cell lacks the system for correction of mutations that may appear in the viral RNA. The loss of large fragments of viral genomes could guarantee the safety of such vaccines; however, this would decrease considerably the viability of mutant viruses.
Note that a high pathogenicity of flaviviruses as well as of many other pathogens complicates the research into these agents; this entails stringent biosafety measures and a high price of the viral preparations. From this standpoint, the use of noninfectious DNA copies of TBEV genome is a very promising direction.
The Institute of Chemical Biology and Fundamental Medicine succeeded in constructing a number of genetically engineered DNA, in particular, containing a full-size DNA copy of tick-borne encephalitis virus (Dobrikova et al., 1996). Construction of such stable DNA copies of viral genomes opens wide possibilities for studies into the virus replication in cells as well as functions of individual proteins and their complexes.
Immunization with Genes
A new approach to prevention of infectious diseases—gene immunization—appeared in 1993; this approach implies a direct introduction into the organism of genetically engineered DNA, i. e., the recombinant plasmids (carrier vectors) that do not contain the entire genome of an infectious agent but its individual genes.
Researchers from the Institute of Chemical Biology and Fundamental Medicine constructed four plasmids of this type, each carrying various genes of a TBEV strain. To assess the effect of gene immunization, vaccinated mice were challenged with lethal doses of the same TBEV strain. It was found that some of the plasmids caused a certain protective effect.
Scientists propose to use the genetically engineered plasmids carrying individual viral genes as vaccines against viral diseasesTo study the potential undesirable effects of such vaccines, the plasmids were introduced into various cell cultures. It turned out that if the cells were cultivated for several months, the plasmids were essentially modified and inserted into the host genome.
Thus, despite the positive results of gene vaccination, the application of DNA vaccines is yet questionable, as the safety issues are an obstacle to their use. Possibly, this obstacle may be bypassed by designing the RNA vaccines with analogous action, as there is no risk of integration into the DNA genome of the host cell in this case. However, these are the goals for future research.
How can we protect ourselves from tick-borne encephalitis? The simplest method was proposed back in the 1930s by Zilber — “Keep away from ticks!” The people whose occupation requires a long stay in the forest should be vaccinated in strict adherence to the immunization scheme. In this case, the illness develops extremely rarely and, as a rule, in a milder form.
Is it possible to get rid of encephalitis completely, for example, by eradicating its main vectors, ixodid ticks? Once this attempt was made with the help of the notorious DDT; however, the consequences of mass treatment of forests with this strongest toxin were indeed horrible.
Perhaps, it is possible to eradicate tick-borne encephalitis virus, but only by wiping out all the living things, as the natural cycle of this virus involves a countless number of living organisms. Thus, people should take the existence of TBEV in nature as a matter of course, nonetheless continuing to develop the necessary tools to defend themselves from this dangerous enemy.
*For the structure and properties of RNA, see SCIENCE First Hand, no. 2, 2004.
**For PCR method, see SCIENCE First Hand, no. 1, 2006.