Tick-Borne Encephalitis
Nordic temperament, merciless towards humans
Ixodid ticks are widely known as carriers of pathogens of many natural focal infections that can be transmitted to humans through the bite. The tick threat cannot be fully eradicated for several reasons, and no radical solution has yet been provided to protect against the infectious agents carried by ticks. Among these pathogens, the greatest danger comes from the virus of tick-borne encephalitis. This disease currently presents a challenge for Russia, where the most severe, even lethal, forms of it occur. For this reason, scientists continue their vigorous efforts to investigate the causative agent of this disease, which opens up the potential for creating means of early diagnosis, prevention, and targeted therapy for this formidable tick-borne infection
Tick-borne viral encephalitis is a disease that causes damage to the central nervous system in humans. It is a so-called natural focal infection; i. e., the encephalitis virus circulates in nature, being carried by wild animals. Its major natural reservoir is small mammals, in whose bodies it can persist and multiply even without visible signs of disease.

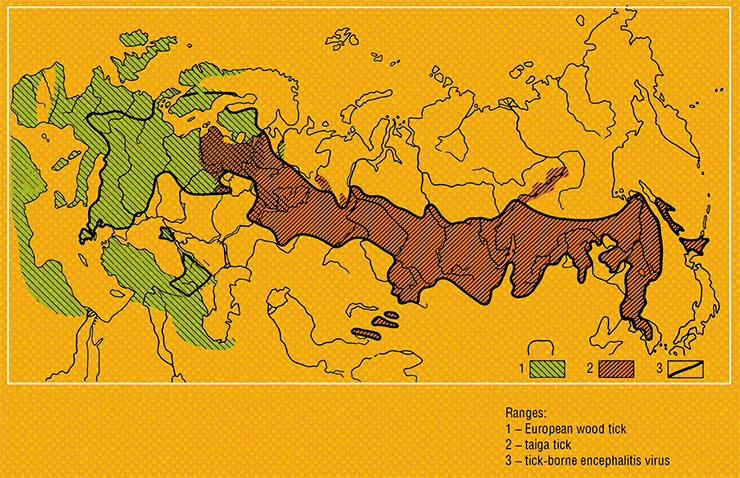
The range of the taiga tick lies almost entirely within the boundaries of Russia and covers middle and southern taiga, coniferous–deciduous and deciduous forests, and forest steppe. The range of the European wood tick extends across almost all of Western and Central Europe; this tick also occurs in limited quantities in Africa, Crimea, and the Middle East (Mediko-geograficheskii atlas Rossii, 2015). A substantial part of the European Russia hosts both of the tick species simultaneously
In the vast majority of cases, tick-borne encephalitis is transmitted by a vector (i. e., via the vector-borne route). In Russia, its main vectors and “keepers” are ixodid ticks, primarily the taiga tick Ixodes persulcatus and the wood tick Ixodes ricinus, whose distribution virtually coincides with the territory with tick-borne encephalitis record. However, alternative cases of infection are described, i. e., through the consumption of unboiled goat and sheep milk or dairy products and (very rarely) via cow milk (Ruzek et al., 2019; Korenberg et al., 2013). Theoretically, one cannot rule out the possibility of infection as a result of crushing a tick, whereby the virus gets onto mucous membranes or damaged skin.
In Russia, ticks bite about half a million people every year, and more than two thousands of those bitten by ticks contract tick-borne encephalitis. Without timely treatment, mortality can reach 20–25 %, and in any case – whether treated timely or not – 10–20 % of the patients may develop lifelong neurological complications.
Tick-borne encephalitis: from mild to lethal
Tick-borne encephalitis can run its course in different ways: from asymptomatic, or mild, forms to severe, even lethal, ones. The incubation period usually lasts one to two weeks, but cases are known of both transient (2–3 days) and delayed (up to 1 month) onset of the disease.
This infection usually develops in two stages. At first, the virus spreads systemically throughout the body, which process is accompanied by fever and such symptoms as headache, fatigue, muscle pain, nausea and/or vomiting. For those “lucky ones,” in whom the virus does not penetrate into the central nervous system, that is all. The other patients enter the second, neurological stage of the disease, characterized by impaired consciousness, flaccid paralysis (a drop in muscle tone), and paresis of the cervicobrachial musculature.
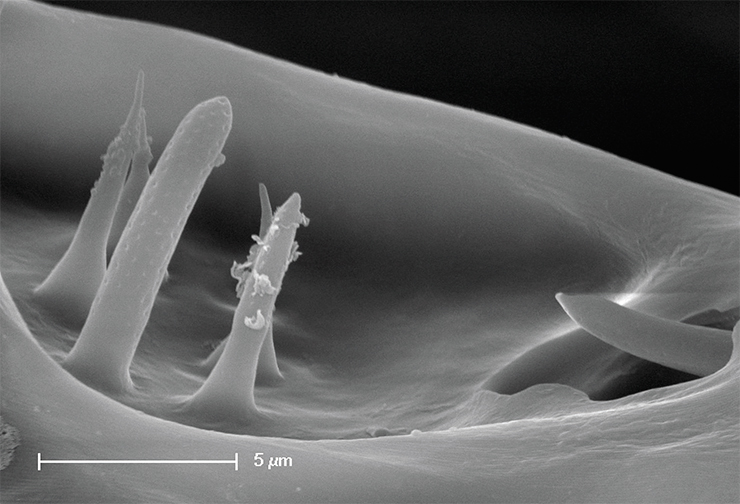
Some patients show signs described as chronic tick-borne encephalitis. This form of the disease is recorded in Russia only, where it reaches 1–3 % of all the cases of tick-borne encephalitis, and is virtually absent in Europe. Focal lesions of the central nervous system in such patients manifest themselves later, i. e., weeks or months after the body temperature decreases to a normal level, which usually serves as a sign of recovery.
TICKS’ NEIGHBORS Small mammals – rodents and, to a lesser extent, insectivores – serve as main hosts for the young of ixodid ticks, i. e., larvae and nymphs. They also serve as reservoir hosts for the TBEV.Other wild mammals, including artiodactyls (roe deer, deer, moose, wild boars), foxes, and hares, provide “food and home” for both nymphs and adult ticks. Some animals are found to carry on their bodies a huge number of ticks, including those infected with the TBEV, and to have specific antibodies in the blood.
Several ixodid tick species also attack birds; moreover, migratory birds can carry ticks over long distances. The blood of tick-infected birds is found to contain both the virus itself and antibodies to it. It seems that during their migrations, birds maintain stepwise (through stopovers) transmission of infected ticks, which leads to the formation of local infection foci.
Importantly, not a single reliable case of clinical manifestations of tick-borne encephalitis has been described among all these wild mammals and birds
Latent chronic infection can manifest itself months or even years after under circumstances such as hypothermia, injury, hard physical labor, alcohol intoxication, abortion or childbirth, or even physical therapy. In other cases, it manifests itself as continuously progressing focal lesions of the central nervous system and ends in death.
An early sign of chronic infection is headache, which intensifies as the disease progresses. The course of the chronic disease lasts 1 to 20 years, or more, and in 17 % of cases, the disease ends in death.
As a rule, chronic infection occurs after contraction of the Far Eastern and, as believed, especially the Siberian subtypes of the virus (Pogodina, 2005). Meanwhile, a comparison of collection strains of the tick-borne encephalitis virus isolated from patients with acute and chronic forms of the disease revealed no apparent differences in their genomes (Gritsun et al., 2003; Pogodina et al., 2004; Sobolev et al., 2010).
Meet the TBEV
 Now the time has come to present our main character, the tick-borne encephalitis virus (TBEV). Despite the myth that the TBEV emerged only recently (some even blame secret military laboratories, as in the case of tick-borne borreliosis (Vlasov, 2020)), scientists have shown, first by molecular biological methods and then by bioinformatics, that ancestral variants of this pathogen are thousands of years old (Grard et al., 2007; Tkachev et al., 2019).
Now the time has come to present our main character, the tick-borne encephalitis virus (TBEV). Despite the myth that the TBEV emerged only recently (some even blame secret military laboratories, as in the case of tick-borne borreliosis (Vlasov, 2020)), scientists have shown, first by molecular biological methods and then by bioinformatics, that ancestral variants of this pathogen are thousands of years old (Grard et al., 2007; Tkachev et al., 2019).
The 18th-century parish records from the Aland Islands (Finland) contain descriptions of a disease very similar in symptoms to tick-borne encephalitis (Kunz and Heinz, 2003). However, the question about the causative agent had remained unanswered until the first half of the past century, and it was Russian scientists who carried off the palm in finding the answer.
The TBEV was discovered in 1937 in the Far East by an expedition (led by the famous virologist Lev Zilber) commissioned by the USSR People’s Commissariat for Health to an area hit by an epidemic of an unknown disease. Experts identified its carriers, isolated several strains of the pathogen, and obtained antibodies to it. Half a century later, new technology for sequencing nucleic acids and studying proteins allowed researchers to find out how the virus works and to develop effective means for detecting it. As for decoding the viral genome, this was accomplished by researchers from the Siberian Branch of the USSR Academy of Sciences and their Austrian colleagues (Pletnev et al., 1989, Mandl et al., 1989; Pletnev et al., 1990).
SIBERIANS: FIRST AT THE FINISH LINE In the Siberian Branch of the Academy of Sciences, molecular biological studies of the TBEV, including a genome decoding project, were initiated by Mikhail Grachev, a future academician, who in 1984–1987 was head of laboratory at the Novosibirsk Institute of Bioorganic Chemistry (NIBOC), Siberian Branch, USSR Academy of Sciences (now the Institute of Chemical Biology and Fundamental Medicine SB RAS).
In the Siberian Branch of the Academy of Sciences, molecular biological studies of the TBEV, including a genome decoding project, were initiated by Mikhail Grachev, a future academician, who in 1984–1987 was head of laboratory at the Novosibirsk Institute of Bioorganic Chemistry (NIBOC), Siberian Branch, USSR Academy of Sciences (now the Institute of Chemical Biology and Fundamental Medicine SB RAS).
The main works were carried out at the NIBOC, where a group of researchers from Grachev’s laboratory mastered sequencing methods (the group leader was A. G. Pletnev). Research material (the virus itself and DNA copies of the viral genome) were obtained at the Institute of Poliomyelitis and Viral Encephalitis, Academy of Medical Sciences (now the Chumakov Scientific Center for Research and Development of Immune-and-Biological Products). The structure of viral proteins was studied using technologies developed at the Institute of Bioorganic Chemistry (Moscow) and the Special Design Bureau for Analytical Instrumentation, USSR Academy of Sciences.
As a result, the scientists managed to quickly decode the TBEV genome, outstripping their competitors, i. e., Austrian scientists who had been working on a similar project. In the course of pioneering research, the scientists had to overcome many difficulties. For instance, one of the researchers, E. Pressman, who was engaged in the isolation of viral particles, fell ill with tick-borne encephalitis, fortunately, not in the most severe form.
The decoding of the TBEV genome was the first successful Russian project to identify the structure of viral genomes. One of its crucial practical results has been the development of a fast method for virus detection using hybridization of nucleic acids with radioactive DNA probes
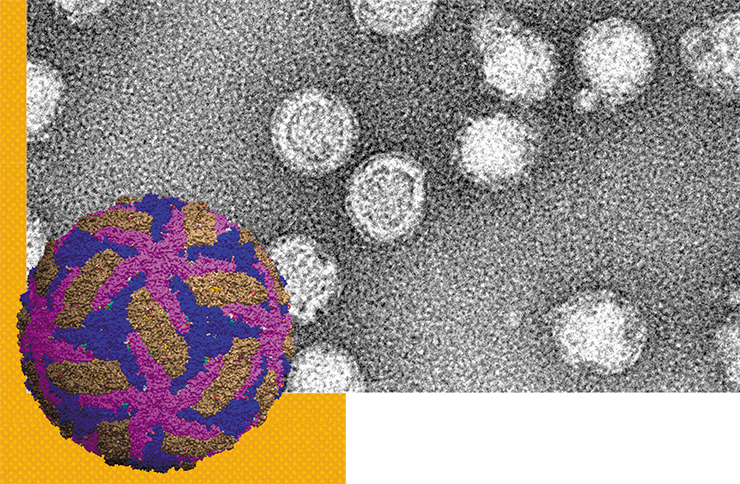
The causative agent of tick-borne encephalitis belongs to the evolutionarily old family of Flaviviridae, which also includes such well-known pathogens as viruses of yellow fever, Japanese encephalitis, Dengue fever, hepatitis C, etc.
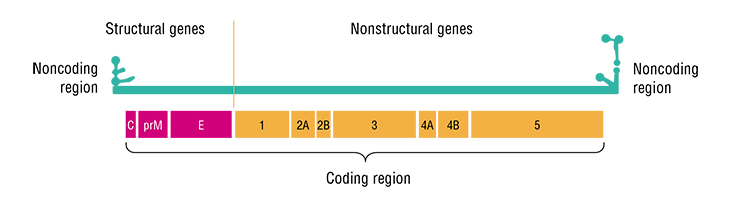
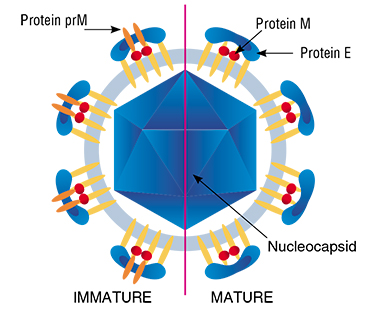
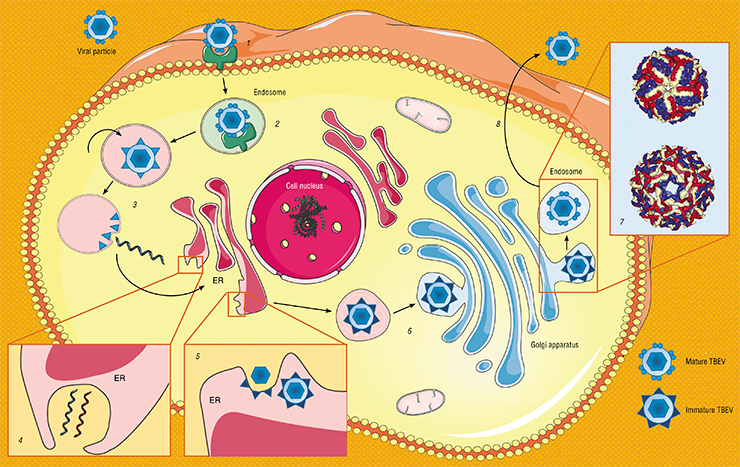
Virions of the TBEV are spherical particles 50 nm in diameter. Their “core,” which conceals the virus’ hereditary material, i. e., single-stranded RNA, is surrounded by a protein – lipid membrane. Two forms of the virions are known: immature and mature, which differ from each other in one of the proteins contained in the envelope. Like in all flaviviruses, the process of intracellular development of the TBEV occurs in the cytoplasm of infected cells, with the participation of viral and cellular enzymes.
Who lives where?
Today’s classifications officially distinguish between three subtypes of the virus: European, Siberian, and Far Eastern. The names are arbitrary, being derived from the names of the regions where the prototype strains were originally isolated. One and the same region may provide record of strains belonging to different subtypes.
Within the eastern branch, it took about 3,000 years for the Siberian and Far Eastern subtypes to form as well as, perhaps, two new subtypes: Baikal and 178–79. Representatives of the western branch spread across Central Europe and further to the British Isles. Some-where along the route, separation oc-curred of the closely related viruses of Spanish sheep encephalitis (in the Iberian Peninsula) and Scottish sheep encephalomyelitis (Heinze et al., 2012; Tkachev et al., 2020)
The European subtype of the TBEV occurs mainly in Western and Eastern Europe as well as in the European part of Russia. However, its range is wider, extending from France and the Netherlands in the west to South Korea in the east. Meanwhile, although more than 80 % of the identified strains in the Baltics belong to this subtype, all the rest are “Siberians” (Dobler and Tkachev, 2019).
In Western and Eastern Siberia, there are descriptions of single strains and isolates of the European subtype of the virus, which differ slightly from the prototype, like the above-mentioned South Korean “Europeans” (Demina et al., 2017; Rar et al., 2017; Kim et al., 2008). Nevertheless, all these strains show a high similarity with the prototype from Europe, which seems surprising since they are separated by a distance of over 4,000 km, and the in-between regions (particularly, the Ural Mountains) show no record of the European subtype of the virus. One of the possible reasons for the emergence of these viral foci in Siberia and the Far East is the seasonal migration of birds carrying infected ticks as well as the deliberate introduction of certain animals that act as carriers.
Nowadays, the TBEV occurs in forest regions throughout Eurasia, from the Atlantic to the Pacific coasts, and its distribution generally coincides with the ranges of the European wood tick and the taiga tick. In recent decades, the TBEV range has been expanding steadily due to the increased economic activity. For example, abandoned forest clearings are overgrown with small shrubs or turn into swamps, which creates an ideal environment for small mammals and the associated ticksThe European subtype pedigree, reconstructed from different studies, is unclear and confusing. An assumption exists that, although this subtype separated from the ancestral form and migrated to the west long time, all the currently known Central European strains emerged only about 300–400 years ago(!). It seems that several migration waves of this pathogen passed through Europe, and analysis of the genetic sequences of different strains demonstrates geographical clustering of the different variants, although this rule does not always apply. A possible explanation lies with changes in the composition of individual populations and entire ecosystems.
Importantly, general population (not geneticists) should know that the European subtype of the TBEV does not cause chronic forms of the disease. In this case, the disease runs its typical biphasic course, and the mortality rate may reach 1–2 %. However, the risk of death is highest in elderly patients, and the death is often caused by an associated bacterial superinfection or neurological lesions, such as paralysis of the respiratory muscles.
Siberians and Far-Easterners
The Far Eastern subtype of the TBEV mainly occurs in the corresponding part of Eurasia, but a record of it exists for other regions too, including the Baltic States, Crimea, Belarus, the European part of Russia, and the Urals and Siberia. In some regions, this pathogen occurs more commonly in urban and suburban areas. The Far Eastern subtype of the virus, known as one of the most pathogenic viruses, can cause different forms of the disease: from subclinical to acute, including lethal, ones (Tkachev et al., 2008; Leonova et al., 2013).
The subtype includes at least four distinct genetic lines. The strains of one of them resemble the Sofjin strain, which was isolated in the Khabarovsk krai from the brain of a patient in 1937 (Zilber, 1939). Today, this line includes strains from the Far East of Russia, Japan, China, Latvia, and the European part of Russia. Strains from the other lines were isolated in Japan, including on the Hokkaido Island, in China, and in the Crimean Peninsula.
This group includes unique and – importantly – dangerous variants. Thus, in 1999, in the southeast of the Novosibirsk oblast (West Siberia), cases were recorded of hemorrhagic forms of tick-borne encephalitis with fatal outcomes (Ternovoi et al., 1999). No previous record of them exists although other tick-transmitted flaviviruses, e. g., the Omsk hemorrhagic fever virus, are known to cause a syndrome associated with increased bleeding of the mucous membranes.
CAUTION! NONPASTEURIZED MILK Tick-borne encephalitis has long been associated exclusively with domestic ruminants (cattle, goats, and sheep) because the virus could be transmitted through milk and through other products made from unpasteurized milk.After infection, these animals do not develop clinical symptoms, but the virus is detected in their blood for up to 19 days. Thus, in one of the regions of Poland, the TBEV was found in sheep (22 %), goat (15 %), and cow milk (11 %) (Dobler et al., 2019). Consumption of these products may lead to the infection of entire population groups, which is periodically observed in the countries of Eastern Europe, Germany, and Russia. In addition, consumption of natural products without heat treatment has been growing in recent years.
The first laboratory-confirmed case of tick-borne encephalitis in horses was reported over 35 years ago. Since then, sporadic publications appeared that described clinical signs of a neurological disorder in horses in Switzerland, Austria, and Germany, presumably caused by the TBEV. Serological studies in Austria showed that about a quarter of all horses have the corresponding antibodies
A fragment of the gene encoding the glycoprotein E of the viral envelope was deciphered to show that this highly pathogenic virus belongs to the Far Eastern subtype; however, several mutations discovered in it had not been described previously. The emergence of such an atypical virus may indicate ongoing evolution in this group of flaviviruses.
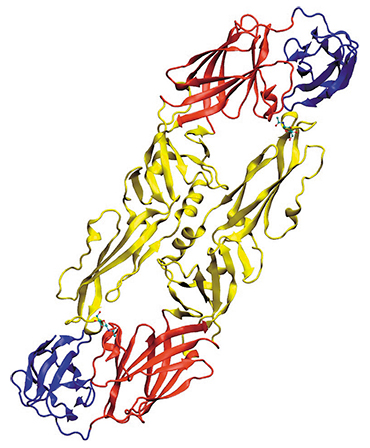 Another example: in 2004, the Glubinnoe/2004 strain was isolated in the Primorsky krai from the brain of a patient deceased from tick-borne encephalitis. Although this strain belongs to the Far Eastern subtype of the virus, it has more than fifty substitutions in the amino acid sequence of protein E in comparison with the known Far Eastern strains, and 14 of these substitutions are unique (Safronov et al., 1991; Goto et al., 2002; Ternovoi et al., 2007). In addition, in the early stages of infection, this strain produces greater quantities of viral particles in cell cultures.
Another example: in 2004, the Glubinnoe/2004 strain was isolated in the Primorsky krai from the brain of a patient deceased from tick-borne encephalitis. Although this strain belongs to the Far Eastern subtype of the virus, it has more than fifty substitutions in the amino acid sequence of protein E in comparison with the known Far Eastern strains, and 14 of these substitutions are unique (Safronov et al., 1991; Goto et al., 2002; Ternovoi et al., 2007). In addition, in the early stages of infection, this strain produces greater quantities of viral particles in cell cultures.
The most widespread subtype is the Siberian one – it occurs almost everywhere throughout the TBEV range. It is believed to cause milder forms of tick-borne encephalitis, compared with the Far Eastern subtype. However, in comparison with the European subtype, infection with the Siberian subtype often leads to the development of chronic forms of the disease, accompanied by neurological and or neuropsychiatric symptoms.
The Siberian subtype is the most heterogeneous one, with the level of similarity between the nucleotide sequences in the coding part of the genome within the subtype of 88 % or higher. Until recently, three genetic lines were distinguished within the subtype, but new studies have yielded two more ones: the Ob and Bosnian lines. Meanwhile, it turned out that the Ob line is the most ancient one among all the Siberians – its ancestors emerged about 4,000 years ago (Tkachev et al., 2017; 2020).
All the genetic lines of the Siberian subtype show a different geographic distribution in Eurasia. The highest genetic diversity is observed in the southern part of West Siberia – it is only the strains of the Bosnian line that do not occur here. This fact likely explains, in part, the high prevalence of tick-borne encephalitis in this region.
In recent years, another three potential subtypes of the virus have been identified. One of them, the Baikal subtype, includes a group of isolates and strains found in Buryatia and in the Irkutsk and Chita oblasts of East Siberia and in the north of Mongolia (Demina et al., 2010; Kozlova et al., 2018). This group of strains forms an independent cluster in the evolutionary tree, and they differ significantly in their genetic structure from the other subtypes.
Studies have shown that these strains are highly virulent and thermally stable. In 2010, a patient died in Mongolia from meningoencephalitis caused by a strain of, presumably, the Baikal subtype. The patient was admitted to hospital on the 11th day after the tick bite and died on the same day. The brain of the deceased showed lesions typical of the most severe forms of acute tick-borne encephalitis (Khasnatinov et al., 2010). A single isolate of this subtype was also found in West Siberia in the tick Ixodes pavlovskyi (Rar et al., 2017).
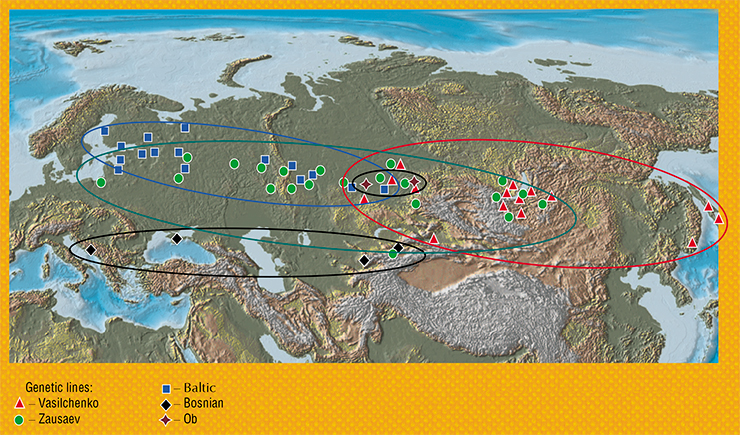
Another two potential subtypes – subtype 178–79 from East Siberia and the Himalayan subtype from the Qinghai – Tibet Plateau in China – differ in their genetic sequence from all the other subtypes by 10–18 % (Demina et al., 2010 Dai et al., 2018). But their record consists of single finds only; therefore, the question of their status remains open.
Diagnosis and prevention
The main danger of tick-borne encephalitis lies in the absence of specific treatment. Like with all other viral diseases, therapy aims at combating the external manifestations of the disease and activating antiviral immunity, and then the body must cope with the infection itself. The virus is also dangerous because it can cause a chronic form of the disease, possibly leading to paralysis and disability. Therefore, it is critical to develop methods for the diagnosis and prevention of this disease.
Clinical diagnosis of the TBEV focuses on serological methods to determine the presence of antibodies, i. e., specific protective proteins, or viral particles, in the patient’s blood and on molecular biological methods, which rely mainly on the polymerase chain reaction (PCR) method, capable of diagnosing even tiny amounts of the pathogen.
The most effective prevention for those visiting areas with a high incidence of infected ticks is, firstly, a set of protective measures to avoid tick bites, including protective clothing and chemical products that either repel (repellents) or kill (acaricides) these arthropods. The second method is vaccination, which is currently the only effective way of prevention against tick-borne encephalitis.
Thus, among the tick-borne encephalitis patients admitted to the Infectious Diseases Clinical Hospital No. 1 of Novosibirsk in the epidemiological season 2017–2018, all those with a severe course of the disease were unvaccinated.
The first vaccine against tick-borne encephalitis was developed in the Soviet Union as a formaldehyde-inactivated suspension of the brain of mice infected with the Sofjin strain. In 1939, it successfully passed clinical trials. Since 1958, a modified variant of this vaccine has been used in all the regions with incidence of tick-borne encephalitis. By the end of the 1980s, a vaccine was developed on the basis of an inactivated highly purified concentrated virus produced in cell cultures. Now in Russia, three vaccines are manufactured, including variants for children from an early age.

However, most dogs do not deve-lop any pronounced clinical signs of the disease. Nevertheless, if they do appear, the animal dies in 16–50 % cases within the first week of the disease. Most common is the acute form, which ends in complete remission in one to two weeks; less frequent is a long course of the disease – these animals often suffer subsequently from paresis, muscle atrophy, epileptic seizures, or blindness.
"Tick-borne encephalitis has been described mainly in adult dogs of medium to large breeds, such as Rottweilers and Huskies, but no data are available to predict the susceptibility of a given animal to this infection. There is neither specific treatment nor a licensed prophylactic vaccine for tick-borne encephalitis in dogs. However, dogs are known to develop specific antibodies in response to the human vaccine.
An important preventive measure is to protect dogs from ixodid ticks (which, by the way, carry borrelia, which cause disease in dogs, and piroplasmosis pathogens, which are especially dangerous for these animals) mainly through regular acaricidal treatment and the immediate removal of all ticks found on the dog
All the Russian vaccines based on the highly pathogenic Far Eastern subtype reliably protect against infection or contribute to an easier course of the disease. The two European vaccines, produced in Austria and Germany, are based on an inactivated virus of the European subtype, which typically causes a mild form of the disease.
Emergency prevention in the case of an infected tick bite in an unvaccinated patient is to inject antitick immunoglobulin products, i. e., a concentrated solution of a purified fraction of immune proteins (antibodies) isolated from the plasma or blood serum of donors immune to this disease. When administered within the first three days after a tick bite, this drug is believed to reduce the likelihood of infection.
However, this method is much less effective than vaccination. Moreover, there is a risk of negative side effects from the administration of a drug obtained from donated blood. Thus, the European Union has abandoned the use of antitick immunoglobulin due to the possible development of the so-called antibody-dependent increase in infection – this phenomenon still requires further study (Ruzek et al., 2019).
In Russia, however, the use of antitick immunoglobulin is justified because Siberia and the Far East are known to have the most dangerous strains of the pathogen. But there is also good news – the Institute of Chemical Biology and Fundamental Medicine SB RAS (Novosibirsk) has developed a new product that makes it possible to abandon the use of donor blood components of humans or animals.
Scientists embedded into the human antibody a mouse antibody fragment that tightly binds the viral particle, thus obtaining a protein that effectively neutralizes the pathogen. Such hybrid antibodies can be produced using standard biotechnological methods. Preclinical trials of the new biotechnological drug have been successful; i. e., the pure “antibodies” successfully protected infected laboratory mice from TBEVs of the Far Eastern, Siberian, and European subtypes. Thus, there is a chance that in the near future, tick-borne encephalitis may become less threatening to human lives.
Although tick-borne encephalitis has been thoroughly studied for decades, its incidence in some parts of Russia, especially in Siberia, remains high. Tick-borne encephalitis remains one of the most socially significant natural focal viral infections in our country, given that we have a widespread incidence of especially pathogenic strains of the virus.
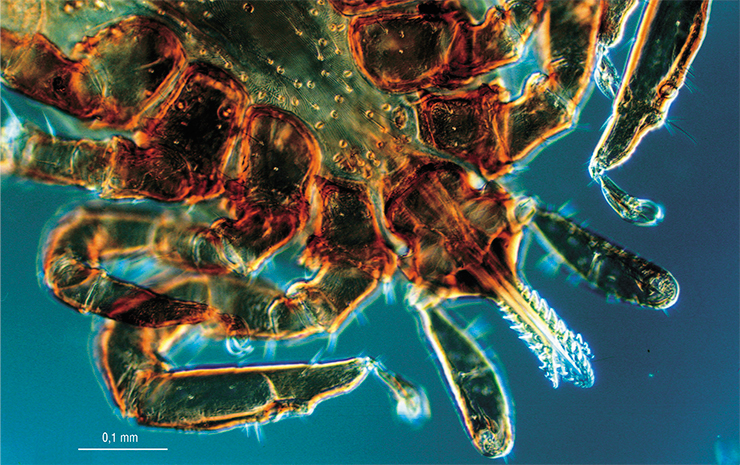
Recent studies have revealed new genetic variants of the virus that cause severe forms of the disease in humans and may go undetected by modern diagnostic test systems. For this reason, it is extremely important to continue research on the genetic diversity of the pathogens in order to improve the existing and develop new diagnostic systems to detect a wide range of genetic modifications of the pathogen. Moreover, the detection of pathogenic regions in the viral genome may allow us in the future to develop diagnostic tools for predicting the severity of the disease in a person bitten by an infected tick.
Based on the results of genetic studies of the TBEV, one can now conduct monitoring surveys in order to identify regions with a high risk of human infection and to assess in advance the severity of the disease in a given region.
By the way, vaccination remains so far the only reliable way to prevent tick-borne encephalitis. Meanwhile, one is now increasingly asking the question to what extent the strains underlying all the modern antiviral vaccines match the actual genetic variants of the pathogens. It is only regular genetic monitoring that can yield an answer to this question, as clearly shown by the example of the new coronavirus.
References
Dobler G., Erber W., Bröker M., Schmitt H. J., eds. The TBE Book. 2nd ed. Singapore: Global Health Press, 2019. DOI: 10.33442/978-981-14-0914-1_11.
Ierusalimskii A. P. Kleshchevoi entsefalit. Rukovodstvo dlya vrachei (Tick-Borne Encephalitis. A Guide for Doctors), Novosibirsk, 2001 [in Russian].
Korenberg E. I., Pomelova V. G., Osin N. S. Prirodnoochagovye infektsii, peredayushchiesya iksodovymi kleshchami (Zoonotic Infections Transmitted by Ixodid Ticks). Moscow, 2013 [in Russian].
Pogodina V. V., Frolova M. P., and Erman B. A. Khronicheskii kleshchevoi entsefalit. Etiologiya, immunologiya, patogenez (Chronic Tick-Borne Encephalitis. Etiology, Immunology, Pathogenesis). Novosibirsk: Nauka, 1986 [in Russian].
Somova L. M., Frolova M. P., Pogodina V. V. et al. Patologiya neiroinfektsii, vyzyvaemykh virusami kompleksa kleshchevogo entsefalita (monografiya-atlas) (Pathology of Neuroinfections Caused by Tick-Borne Encephalitis Viruses (Monograph & Atlas)). Moscow: Sinteriya, 2018 [in Russian].
Votyakov V. I., Zlobin V. I., and Mishaeva N. P. Kleshchevye entsefality Evrazii (voprosy ekologii, molekulyarnoi epidemiologii, nozologii, evolyutsii) (Tick-Borne Encephalites in Eurasia (Ecology, Molecular Epidemiology, Nosology, Evolution)). Novosibirsk: Nauka, 2002 [in Russian].
Yakimenko V. V., Mal’kova M. G., Tyulbko Zh. S. et al. Transmissivnye virusnye infektsii Zapadnoi Sibiri (regional’nye aspekty epidemiologii, ekologii vozbuditelei i voprosy mikroevolyutsii) (Vector-Borne Viral Infections of Western Siberia (Regional Aspects of Epidemiology, Pathogen Ecology, and Microevolution)). Omsk: KAN, 2019 [in Russian].
Zilber L. Vesennii (vesenne-letnii) epidemicheskii kleshchevoi entsefalit (Spring (spring–summer) epidemic tick-borne encephalitis) // Arkhiv Biol. Nauk. 1939. Vol. 56(2) [in Russian].
Zlobin V. I., Belikov S. I., Dzhioev Yu. P. et al. Molekulyarnaya epidemiologiya kleshchevogo entsefalita (Molecular Epidemiology of Tick-Borne Encephalitis). Irkutsk, 2003 [in Russian].