Tick-Borne Rickettsioses: Close Relatives of Typhus
If you have the bad luck to fall “prey” to a blood-sucking tick, you are at risk of contracting rickettsiosis, one of the many tick-borne infections. The most notorious such infections cause tick-borne encephalitis and Lyme disease, but one clearly underestimates the danger associated with other tick-borne pathogens such as rickettsia. These relatives of typhus are by no means harmless, and rickettsioses rank second in incidence among bacterial tick-borne infections in the Asian part of Russia. In contrast to North America and Europe, deaths in the case of infection with rickettsiae are rare in Russia. Nevertheless, tick-borne rickettsioses may occur in severe and sometimes atypical forms, and they require hospitalization and adequate treatment
 At the end of the 19th century, settlers in the piedmont of the Rocky Mountains of Montana were hit by a most severe outbreak of an unknown disease with high fever, hemorrhagic rash, and other acute symptoms; mortality among the patients reached 20–30 %. In the first decade of the 20th century, Howard Taylor Ricketts, a pathologist and one of the first infectious disease specialists in the United States, established that the human infection occurred as a result of a bite by blood-sucking ixodid ticks of the genus Dermacentor. He analyzed the blood of the patients to find tiny bacterium-like microorganisms, and he proved experimentally on guinea pigs and monkeys that the infection can be transmitted through infected blood.
At the end of the 19th century, settlers in the piedmont of the Rocky Mountains of Montana were hit by a most severe outbreak of an unknown disease with high fever, hemorrhagic rash, and other acute symptoms; mortality among the patients reached 20–30 %. In the first decade of the 20th century, Howard Taylor Ricketts, a pathologist and one of the first infectious disease specialists in the United States, established that the human infection occurred as a result of a bite by blood-sucking ixodid ticks of the genus Dermacentor. He analyzed the blood of the patients to find tiny bacterium-like microorganisms, and he proved experimentally on guinea pigs and monkeys that the infection can be transmitted through infected blood.
The disease was called Rocky Mountain spotted fever (RMSF) although it later proved to occur almost everywhere in North America as well as some parts of South America. In 1910, Ricketts engaged in a study of an unknown disease in Mexico, which turned out to be typhus, and revealed similarities both between the symptoms and the causative agents of this disease and the tick-borne fever. During these studies, the 39-year-old professor contracted typhus and died.
In 1916, the term rickettsia was first used by the Brazilian microbiologist and infectious disease specialist Henrique da Rocha Lima, the founder of the theory of rickettsioses. Over time, this term was assigned to the entire group of similar microorganisms, which includes pathogens of both tick-borne spotted and typhoid fevers carried by lice and fleas.
In Russia, the first cases of tick-borne rickettsiosis were recorded in 1934–1936 in the Krasnoyarsk region and in the Far East, at about the same time when the first records of spring outbreaks of tick-borne encephalitis appeared there. It was a time of vigorous development of the eastern regions of Russia, and epidemics of unknown diseases attracted special attention.
Later, research expeditions accurately identified the nature of the infection and isolated its pathogen, which was called Dermacentor oxenus sibirica (subsequently renamed as Rickettsia sibirica). The disease itself was named North Asian tick-borne rickettsiosis, or Siberian tick-borne typhus (Loban, 2002).

This disease is tied to specific territories, or natural foci. In Russia, they exist in Siberia and the Far East, as well as in neighboring countries: Turkmenistan, Armenia, Kazakhstan, and Mongolia. However, the role of vertebrates as a reservoir of infection seems to be insignificant. In fact, rickettsiae are very rarely found in rodents, unlike pathogens of other tick-borne diseases. For humans, the situation is the same as with other tick-borne infections – they may contract rickettsiae only by accident, when bitten by an infected tick.
Bacterial “relatives” of mitochondria
Rickettsiae are among the smallest bacteria – their length is 1–2 microns or less – comparable in size with large viruses. Moreover, these bacteria cannot grow on nutrient media, which is not surprising as they are exclusively intracellular parasites and can only multiply in the cells of living organisms.
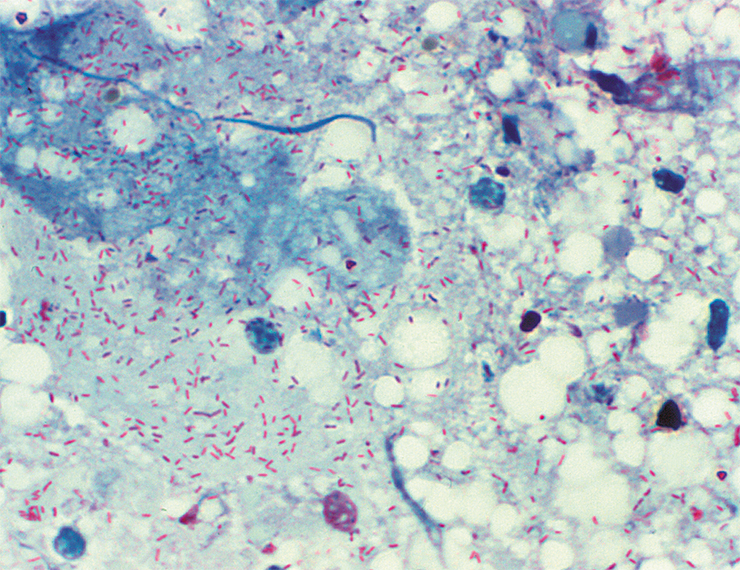
The causative agent of Rocky Mountain spotted fever (RMSF) was called Rickettsia rickettsii in honor of its discoverer Howard Taylor Ricketts. His name was also memorialized in the terminology for the higher taxa, i.e., the family and order.
The term rickettsia was first used for the causative agent of typhus by Henrique da Rocha Lima, the head of the Pathology Department at the Tropical Institute in Hamburg. This pathogen was identified in 1913 in the body and feces of lice by his colleague and friend, the Czech microbiologist Stanislaus von Prowazek. In 1915, they worked together in a camp hospital in Chotebuz, where a typhus epidemic broke out among Russian prisoners. Prowazek got infected and died despite good care and a passionate desire to overcome the disease. When Rocha Lima later described the causative agent of typhus, he named it Rickettsia prowazekii in honor of the two researchers who died while studying this dangerous disease. By the way, the fact that lice may be involved in the transmission of typhus was first suggested in 1908 by the Russian scientist Nikolay Gamaleya and was experimentally confirmed a year later by Charles Nicolle (France), who received the Nobel Prize for that discovery
The fact that rickettsiae prefer a life of safety and comfort agrees well with the opinion that these bacteria are indeed the closest ones evolutionarily to the extinct microorganisms that became the progenitors of mitochondria, the crucial intracellular organelles. These structures produce a molecule called ATP, the universal source of energy for cells. Mitochondria have their own genome, which is passed down from generation to generation (including in humans) through the maternal line.

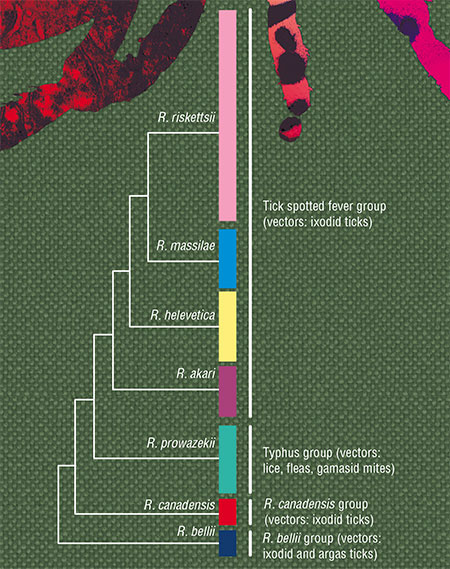
At present, the genus Rickettsia unites more than 40 species, which are divided into several groups and subgroups. The species that are pathogenic for humans are part of two main groups: typhus (vectors: lice and fleas) and tick-borne spotted fevers (vectors: ticks). As for the remaining groups, their danger to humans is yet to be investigated.
Researchers have deciphered the genomes of the currently known species of rickettsiae. Represented by a single ring chromosome, they are small compared to the genomes of free-living bacteria, which is generally typical of intracellular parasites: why have a large genome when one’s living is fully procured by the host organism? Genomic rearrangements are also rare in rickettsiae because at such a size, any changes may have disastrous consequences.
However small the number of genes in rickettsiae, they had borrowed some of them from other organisms. For example, the genome of R. felis contains more than one and a half hundred genes from other bacteria and even higher organisms (eukaryotes).
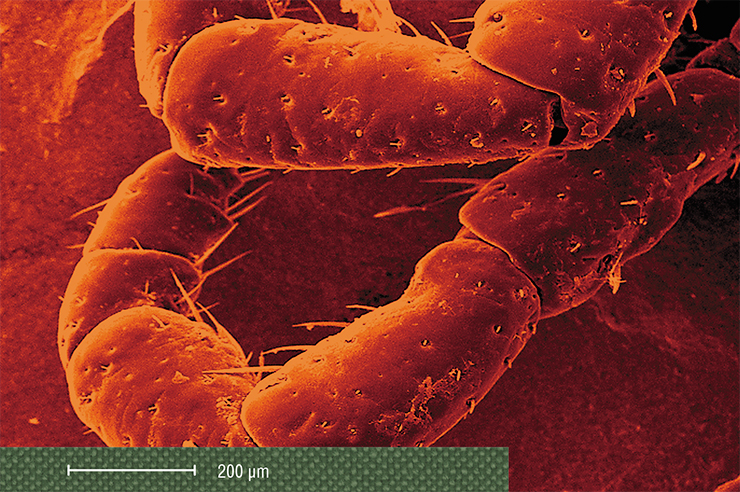
TYPHUS: EPIDEMIC AND ENDEMIC
The typhus group includes the causative agents of epidemic typhus (vectors: lice), Rickettsia prowazekii, and of endemic typhus (vectors: fleas and rats), Rickettsia typhi.
Epidemic typhus is characterized by a cyclic course with fever, rash, and acute damage to the nervous and cardiovascular systems. Its source is always a sick person; body or head lice act as a vector. People get infected through rubbing louse feces into the skin by scratching or through the mucous membranes of their eyes and respiratory tract (Rudakov et al., 2016). The infection is not transmitted through lice bites since the pathogen multiplies only in the epithelial cells of the insect’s intestines (Loban et al., 2002).
Endemic typhus is characterized by a cyclic course with a roseolous papulous skin rash; the disease is mostly benign with low mortality. The reservoirs of infection are rats and house mice; the vectors are fleas, three species of lice, and several species of gamasid mites. Transmission to humans occurs via the feces–inhalation–contact route (Loban et al., 2002). The infection is found on all the continents except Antarctica
The loss of some regulatory genes can change the pathogenicity of a bacterium, with either direction of change being possible. For example, R. prowazekii, one of the most pathogenic rickettsiae, has the smallest genome. But rickettsiae may also lose the genes that make them deleterious.
The genome of some rickettsiae also contains plasmids, i. e., free genetic elements that can be inherited relatively independently and even transmitted from one bacterium to another during conjugation (a bacterial analog of the sexual process). However, little is known about the role of plasmids in the life of rickettsiae.
Ab ovo
Of the 36 rickettsia species in the tick-borne spotted fever group, only 16 are dangerous to humans. Tick-borne rickettsiosis is transmitted through a bite of an infected tick. An exception is R. felis, which is carried by fleas. In cats, R. felis causes fever; in humans, it causes symptoms quite typical of rickettsioses, with possible neurological disorders.
In the body of the tick itself, rickettsia can be found almost everywhere, including the salivary glands and ovaries. Therefore, they not only remain in a tick throughout its life, from the larva to imago (adult), but also can be very effectively transmitted through the egg to its offspring (Rudakov et al., 2016). In this respect, rickettsiae stand out from other infectious agents such as tick-borne encephalitis virus or borreliae.
Typhus epidemics during the wars claimed the lives of millions of people as the mortality rate reached 10 %. An example is the catastrophic situation during the Civil War in Russia in the early 20th century. A terrible epidemic of typhus broke out in the city of Novonikolaevsk (today’s Novosibirsk) in the winter of 1919–1920; more than sixty thousand soldiers and local residents died from the disease. The overall picture was that of a pestilence rather than an epidemic, and the goal and efforts of Gubchekatif (i. e., the local emergency committee on typhus) were to turn the pestilence into an epidemic. Typhoid epidemics in the city of Novonikolaevsk ended only in 1922–1923.
In Russia, the last outbreak of typhus was recorded in 1998 in a psycho–neurological hospital in the Lipetsk region. Currently, infection foci exist in less developed countries with low living standards in South and Central America and in Africa
Since the pathogen is passed down through generations, the infestation in some species of ixodid ticks can be as high as 70–80 %! Therefore, bites of larvae and nymphs, as well as adult ticks, are dangerous for humans. Larvae often attack children and can go unnoticed due to their small size, making it difficult to diagnose the disease in an infected patient.
Some species of rickettsiae gravitate towards their “own” tick species; knowing these features is very helpful when assessing the epidemiological situation in different regions.
Thus, in the Asian part of Russia, the following rickettsia species most commonly occur in ticks: Candidatus R. tarasevichiae, R. raoultii, R. helvetica, R. sibirica, and R. heilongjiangensis (the last two species represent the greatest danger to humans). In addition, e. g., R. raoultii and R. sibirica clearly prefer ticks of the genus Dermacentor (Igolkina et al., 2018a; Mediannikov et al., 2006; Shpynov et al., 2006).
The geography of tick habitats may also affect this distribution in unexpected ways. Thus, although taiga ticks most often carry Candidatus R. tarasevichiae, more than 60 % of these ticks on Sakhalin Island are infected with R. helvetica, a completely different rickettsia. This difference is probably due to the geographical isolation of the island.
Astrakhan, Siberia, and the Far East
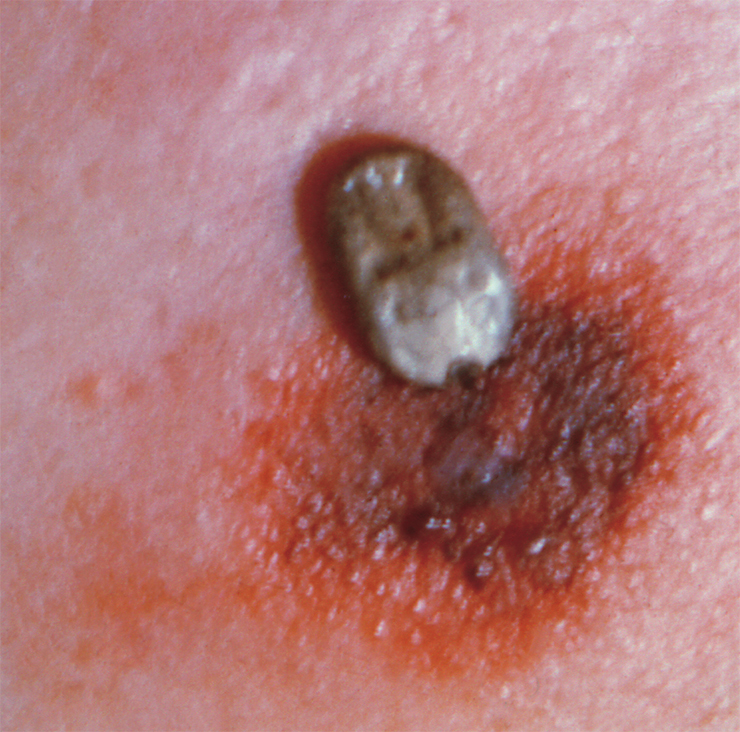
Most of rickettsioses manifest themselves in the same typical symptoms: high fever, spotty skin rash, swollen lymph nodes near the bite site. At the site of tick suction, a sore often appears, covered with a dark crust and surrounded by a patch of reddened, inflamed skin. The patient may suffer from muscle- and headaches, lethargy, apathy, sleep disturbances, and in rare cases, neurological disorders.
In addition, rickettsioses caused by different pathogens may have their own specific features and differ in severity. RMSF is one of the gravest diseases – mortality reaches 4 % even with timely treatment. Mediterranean spotted fever is widespread in Europe, with a mortality rate of up to 2.5 %.
Rickettsioses that occur in Russia follow a milder course. Officially, only two types of tick-borne rickettsiosis are registered in the Russian Federation: Astrakhan spotted fever in the European part, mainly the Astrakhan region (20 cases per 100,000 people) (Rudakov, 2016), and the aforementioned Siberian tick-borne typhus in the Asian part of Russia. Most cases of the disease east of the Urals were recorded in the Altai krai and the Altai Republic (up to 130 cases per 100,000 people).
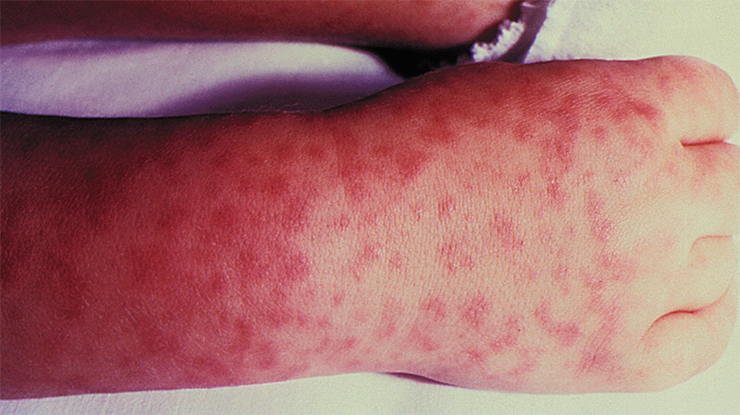
People can contract tick-borne fevers through a bite of an infected tick, when rickettsiae get into the body with the tick’s saliva. The incubation period averages three to seven days. Bacteria first multiply at the bite site (which is why a crust forms at the site, i.e., the so-called primary affect); then they get into regional lymph nodes (causing lymphadenitis and/or lymphadenopathy) and then into the blood (Rudakov, 2016). The rash appears on the second to fifth day of the disease. Rickettsiae predominantly affect endothelial cells (the inner lining) of blood vessels but may affect liver cells (hepatocytes) and macrophage immune cells
Although it is believed that only one tick-borne rickettsiosis, caused by R. sibirica, occurs in the Asian part of Russia, it was recently found that the causative agent in the Khabarovsk region is most often R. heilongjiangensis (Mediannikov et al., 2006). The disease even received a separate name: Far Eastern tick-borne rickettsiosis.
The causative agents of rickettsioses can only be discriminated using molecular genetic methods. Thus, it was shown that R. sibirica is indeed the main infectious agent in the Novosibirsk region and in Altai (Igolkina et al., 2018b; Granitov et al., 2015), but research data for the other regions of West and East Siberia are yet to be collected.
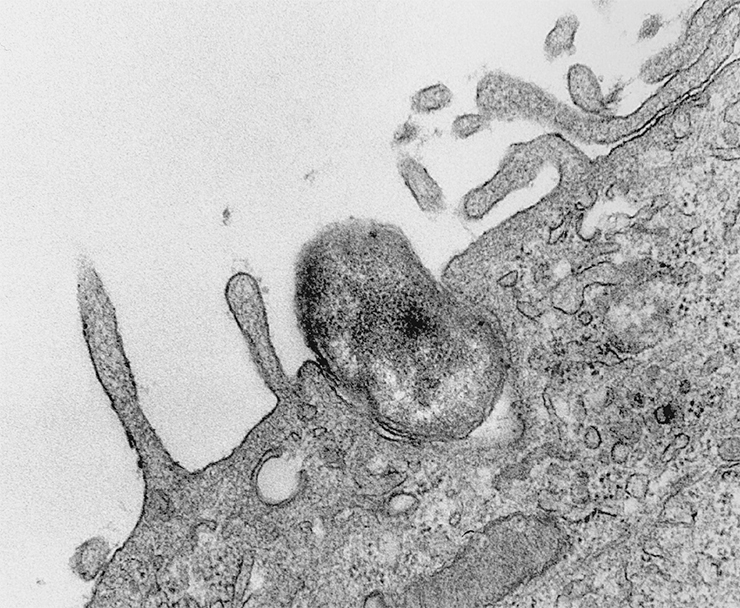
Since rickettsiosis may end in death, even in Russia, timely diagnosis and treatment are critical. Currently, the disease is diagnosed from its clinical manifestations and treated with tetracycline antibiotics (e. g., doxycycline). Rickettsiae are also sensitive to chloramphenicols (levomycetin) (Rudakov, 2016). Recovered patients develop strong immunity, which works against all tick-borne rickettsioses regardless of the pathogen. No relapses are observed.
However, things are not all that shiny.
Atypical infections
Recently, in different countries, cases have been recorded with unusual symptoms differing from the well-studied “typical” tick-borne rickettsioses. The causative agents in these atypical cases were previously considered nonpathogenic (e. g., R. raoultii, Candidatus R. tarasevichiae, R. helvetica).
 In Russia, the DNA analysis of clinical samples from patients with typical and atypical symptoms from the Novosibirsk region and Altai showed that all the people bitten by the Altai tick were infected with the one and same pathogen, R. sibirica (with one exception).
In Russia, the DNA analysis of clinical samples from patients with typical and atypical symptoms from the Novosibirsk region and Altai showed that all the people bitten by the Altai tick were infected with the one and same pathogen, R. sibirica (with one exception).
In the Novosibirsk region, however, people were infected with a whole bunch of different rickettsiae. The pathogens included the species R. raoultii, R. aeschlimannii, and R. slovaca, and it was the first time that those infections had been recorded within the territory of Russia, while the last two pathogens had not been detected previously in ticks within the Novosibirsk region. Most surprising, however, was the fact that rickettsiae from some of the patients turned out to be new genetic variants that could not be attributed to known species (Igolkina et al., 2018b).
The clinical manifestations of rickettsioses caused by “atypical” pathogens varied markedly. Thus, patients infected with R. raoultii were significantly more likely to have meningeal symptoms, with the most severe forms of the disease observed in elderly and immunodeficient patients. It is possible that these rickettsioses also occur in other regions of Russia, but they cannot be diagnosed from symptoms alone.
The difficulty of diagnosis in atypical cases is further evidenced by the fact that the Novosibirsk patients infected with R. slovaca and new genetic variants of rickettsiae displayed no symptoms typical of rickettsioses.
As concerns Candidatus R. tarasevichiae, there are few cases of rickettsiosis caused by this bacterium although it frequently occurs in ticks. Perhaps, it can only cause infection in immunocompromised people. However, one case was recorded in Russia when a child was simultaneously infected with two types of rickettsiae: Candidatus R. tarasevichiae and R. sibirica. The disease ended in death (Rudakov et al., 2019).
In growing numbers
Modern molecular genetic methods allow researchers to identify not only new genovariants and species of rickettsiae but also their presence in unusual tick species and in new regions.
For example, the rickettsia species R. aeschlimannii, pathogenic for humans, has been discovered for the first time within the territory of the Khabarovsk region in the Far East of Russia; previously, this species was recorded only in the Russian south (the Crimea and the Stavropol region) and, importantly, in ticks of a different genus. The Khabarovsk region is known for another, even more intriguing discovery – researchers found there R. canadensis, a strain that was first isolated from ticks living within the territory of the United States and Canada and later from a single sample obtained in South Korea. The Siberian sample turned out to be genetically identical to the South Korean one, which indicates the presence of a rare “Asian” genovariant of this rickettsia (Igolkina et al., 2018a).
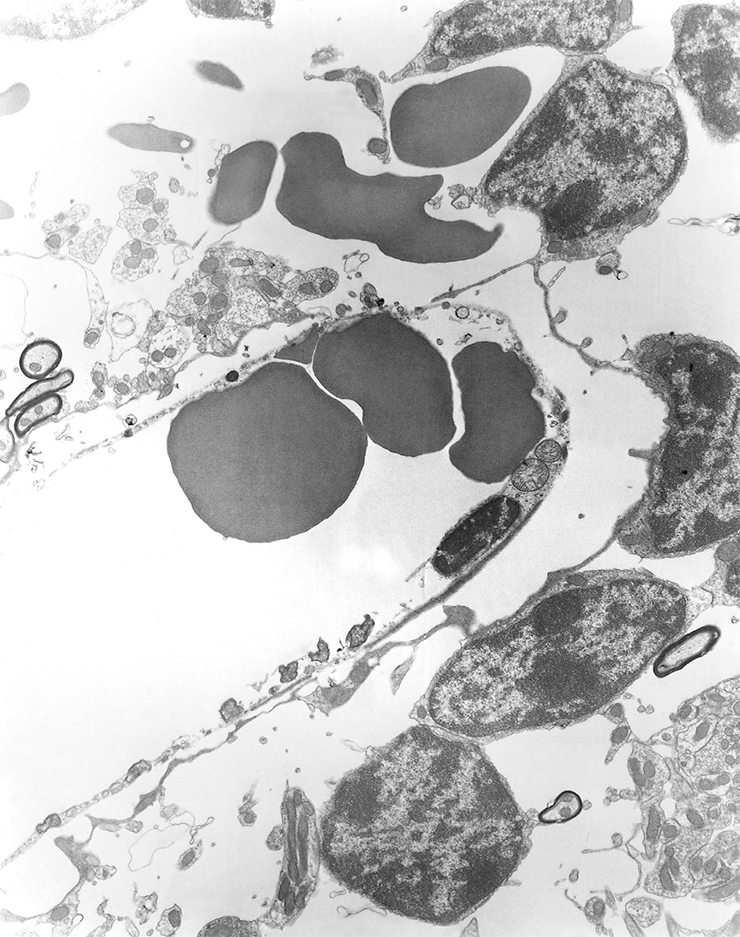
Some rickettsia species, despite their wide distribution, remain in the status of a candidate species, as in the case of the aforementioned Candidatus R. tarasevichiae. They are called so because they have not yet been cultured in the laboratory and properly characterized (Roult et al., 2005).
In this case, it is important to prove that a given rickettsia indeed represents a new species rather than a new genovariant of known species. For example, Novosibirsk researchers have recently characterized (on the basis of four to five genes) two candidate species – Candidatus R. principis and Candidatus R. rara – that were discovered in the early 2000s in the Far East and thus confirmed their status as new species (Mediannikov et al., 2006; Igolkina et al., 2018a).
Information is also updated about the ways of transmission of rickettsiae in nature. Thus, it was assumed that this process may also involve ticks that never attack big mammals and humans. For example, in some areas of West Siberia, burrow ixodid ticks I. trianguliceps and I. apronophorus, which feed on small mammals throughout their entire life cycle, live together with taiga ticks. Thus, larvae and nymphs of taiga ticks can feed on the same animals, which opens up the possibility for exchange in pathogenic species of rickettsiae.
 Researchers from Novosibirsk identified for the first time in burrow ticks the various species of rickettsiae, including candidate ones such as the new Candidatus R. uralica. However, the genovariants of known species found in different ticks were also generally different, suggesting a rather high specificity of the association between burrow ticks and rickettsiae (Igolkina et al., 2015). Based on these data, rickettsiae are unlikely to be transmitted from burrow ticks to other blood-sucking parasites.
Researchers from Novosibirsk identified for the first time in burrow ticks the various species of rickettsiae, including candidate ones such as the new Candidatus R. uralica. However, the genovariants of known species found in different ticks were also generally different, suggesting a rather high specificity of the association between burrow ticks and rickettsiae (Igolkina et al., 2015). Based on these data, rickettsiae are unlikely to be transmitted from burrow ticks to other blood-sucking parasites.
The views on the distribution and pathogenicity of rickettsiae are now beginning to shift. New rickettsia species and, accordingly, new tick-borne rickettsioses are being discovered all over the world. Thus, only two tick-borne rickettsioses are officially diagnosed in Russia, but two new ones have been added “unofficially” to them recently, and this might be only the beginning.
Unlike tick-borne encephalitis and Lyme disease, rickettsioses do not become chronic and do not cause long-term severe health effects. However, they all require treatment, and when the disease manifests itself by poorly expressed or atypical symptoms, it is difficult to make a correct diagnosis. These atypical cases are often associated with rickettsia species that were previously not considered pathogenic.
In complicated cases, the disease can only be accurately diagnosed by molecular genetic or immunological methods; however, in the Russian clinical practice, these methods are virtually nonexistent. Moreover, as of today, Russia has no domestic licensed test systems for detecting rickettsia antibodies in patient sera.
Rickettsiae are quite widespread – in the risk zones, almost every fifth tick appears to be infected – hence the high incidence of disease in people living in the epidemiological foci. Specifically, one of the foci of tick-borne rickettsiosis with the highest incidence rates among the population is the Altai Mountains, a popular holiday destination for Russians. Therefore, in order to improve the accuracy of epidemiological assessments and prevent the spread of these tick-borne infections, it is necessary to continue the studies of the distribution and species diversity both in clinical samples and in nature.
References
Granitov V., Shpynov S., Beshlebova O., et al. New evidence on tick-borne rickettsioses in the Altai region of Russia using primary lesions, serum and blood clots of molecular and serological study // Microbes Infect. 2015. V. 17(11–12). P. 862–865.
Igolkina Y. P., Rar V. A., Yakimen-ko V. V., et al. Genetic variability of Rickettsia spp. in Ixodes persulcatus / Ixodes trianguliceps sympatric areas from Western Siberia, Russia: Identification of a new Candidatus Rickettsia species // Infect. Genet. Evol. 2015. V. 34. P. 88–93.
Igolkina Y., Rar V., Vysochina N., et al. Genetic variability of Rickettsia spp. in Dermacentor and Haemaphysalis ticks from the Russian Far East // Ticks Tick Borne Dis. 2018a. V. 9(6). P. 1594–1603.
Igolkina Y., Krasnova E., Rar V., et al. Detection of causative agents of tick-borne rickettsioses in Western Siberia, Russia: identification of Rickettsia raoultii and Rickettsia sibirica DNA in clinical samples // Clin. Microbiol. Infect. 2018b. V. 24(2). P. 199.e9–199.e12.
Loban K. M., Lobzin Yu. V., and Lukin E P. Rikketsiozy cheloveka: Rukovodstvo dlya vrachei (Human Rickettsioses: A Gide for Physicians), Moscow; St. Petersburg: ELBI, 2002. 473 pp. [in Russian].
Mediannikov O., Sidelnikov Y., Ivanov L., et al. Far eastern tick-borne rickettsiosis: identification of two new cases and tick vector // Ann. N. Y. Acad. Sci. 2006. V. 1078. P. 80–88.
Merhej V., Angelakis E., Socolovschi C. et al., Genotyping, evolution and epidemiological findings of Rickettsia species // Infect. Genet. Evol. 2014. V. 25. P. 122–137.
Raoult D., Fournier P. E., Eremeeva M., et al. Naming of Rickettsiae and rickettsial diseases // Ann. N. Y. Acad. Sci. 2005. V. 1063. P. 1–12.
Rudakov N. V. Rikketsii i rikketsiozy: Rukovodstvo dlya vrachei (Rickettsiae and Rickettsioses: A Gide for Physicians), Omsk: Omsk. Nauch. Vestn., 2016. 424 pp. [in Russian].
Shpynov S., Fournier P. E., Rudakov N., et al. Detection of members of the genera Rickettsia, Anaplasma, and Ehrlichia in ticks collected in the Asiatic part of Russia. // Ann. N. Y. Acad. Sci. 2006. V. 1078. P. 378–383.















