A "Cell" for Cells
Scientists from the Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, and Rzhanov Institute of Semiconductor Physics, Siberian Branch of the Russian Academy of Sciences (Novosibirsk), have developed a new method for isolating minor cell populations from blood and other biological specimens using silicon microchannel chips
The methods allowing populations of viable cells to be rapidly and inexpensively isolated from tissues and biological fluids are in great demand in various fields of cell biology, immunology, and oncology. Of special interest are these methods for minimally invasive medical diagnostics, particularly, for isolating from blood such rare circulating cells as tumor cells in cancer patients or fetal cells in pregnant women. Cytogenetic analysis of isolated cells gives information about chromosome pathologies, while subsequent cultivation and analysis of cellular metabolism provide principally new diagnostic data (for example, on metastatic potential, genetic profile, and drug resistance of cancer cells).
In ordinary cell sorters, light scattering or fluorescence is measured when individual cells pass through a measuring window, and the cells are later sorted based on these data. Recently, microfluidic devices have been proposed for separation of individual cells. Usually such devices are produced by photolithography and consist of a system of microchannels, units for fluid supply and flow control, and a cell detector.
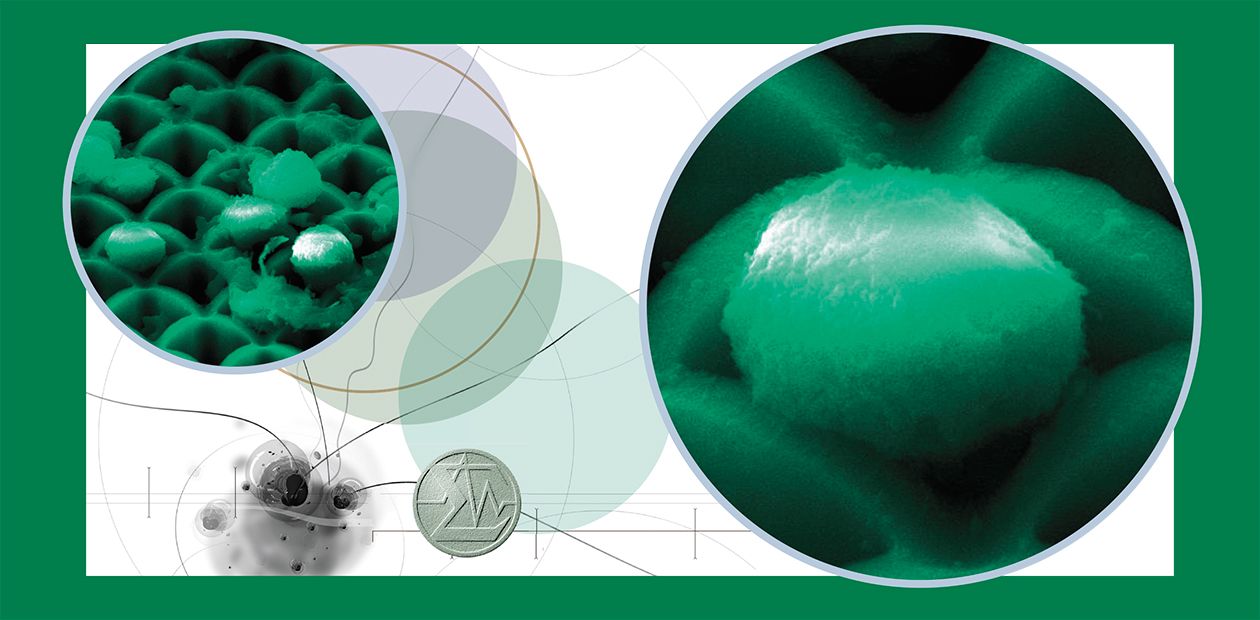
Today, novel technologies for processing silicon materials used in microelectronics make it possible to produce fundamentally new porous structures – silicon microchannel chips (SMCs), which can be used as unique membranes in molecular biology and cell biology experiments.
The technology for SMC manufacture provides for a high-precision control of their porosity, shape and sizes of the sections in end-to-end microchannels (with a cell period of 4—30 microns), and of channel length (from tens to hundreds of microns). The presence of silicon oxide on the surface of such chips allows a covalent immobilization of specific ligands – substances playing the role of “anchors” –on the microchannel walls. Fluidic devices involving SMC sets with various structural characteristics are capable of separating out of heterogeneous cell pools minor target cells which can be of interest for research or diagnostics.
The Institute of Semiconductor Physics, SB RAS, has designed microfluidic devices involving SMCs and selected optimal hydrodynamic conditions for cell separation. The SMCs are produced on the basis of a unique technology which utilizes anodic etching of monocrystalline silicon plates. This method helps to make end-to-end channels of the same section, with parallel walls, and a high purity surface over the entire chip area. The chips display a characteristically high degree of porosity; moreover, the channels, due to specific features of the manufacturing process, widen towards the input surface, which additionally increases their porosity and decreases the resistance to fluid flow.
According to the estimates, at a flow rate of up to 20 ml/ min (through a chip with a porous area diameter of 8 mm), a laminar nonuniform flow is formed in SMC channels, providing a minimal shearing “stress” for the cells. On the other hand, at this flow rate the cells with a size of 2 microns contact at least once with the channel walls and can interact with the adsorbed ligands to cell receptors.
The experimental estimation of hydrodynamic conditions of the cell suspension flow through an SMC has demonstrated that the main resistance to the flow results from the input/output capillaries rather than from the chip itself. It has been found that a differential pressure of 30 millimeters water is optimal for size-selective separation of cells, i. e. the cells whose diameter slightly exceeds the efficient channel radius are not pushed through the chip but remain under it. Such separation conditions neither interfere with the cell membrane integrity nor with the overall cell viability.
An amazing specific feature of SMCs is their ability to “filter” whole blood – an unusual “solution” half of which is cells that are extremely difficult to push even through the pores considerably larger than the diameter of an erythrocyte (its diameter is about 7 microns). Thus, parallel structure and “smoothness” of the channel walls as well as high porosity of the SMC surface makes these structures incomparable cell filters.
Multichannel chips make it possible to separate cells using two characteristic traits: their size and surface structure. For example, cancer cells of epithelial origin are, as a rule, larger than blood cells. In addition, these cells as well as small fetal cells circulating in blood carry specific receptor proteins on their surface; receptor proteins are not found on the surface of normal blood cells. Having immobilized specific antibodies to such receptors on the surface of SMC channels, we can anchor the specific cells in the channels and collect them after washing,
To attach the antibodies to the chip, we should functionalize its surface, i.e. it is necessary to introduce chemically active or marker-specific groups onto the surface. The corresponding methods for functionalizing the SMC surface have been developed and the conditions for covalent attachment of antibodies to the chip surface have been determined at the Institute of Chemical Biology and Fundamental Medicine.
The operational efficiency of such structures was assessed as circulating cancer cells were isolated from the blood of breast cancer patients (their presence is a sign of potential metastases) and embryonic cells, from a mother’s blood. With the help of SMC, circulating tumor cells were isolated from the blood of five of the seven examined cases of a late breast cancer. Fetal cells were detected in the blood of pregnant women (6—7 months of pregnancy) only in three specimens of the eight cases studied. Presumably a higher efficiency in cancer cell isolation results from the fact that the cells were “fixed” on the chip not only by antibodies, but also due to their large size, while the efficiency in isolating fetal cells was limited by the quality of commercial antibodies.
These results demonstrate that SMGs can be quite useful for cell separation. Thus, since this method requires no specialized expensive equipment, it has good prospects for application in practical medicine.
References
Nagrath S., Sequist L. V., Maheswaran S., et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology // Nature. 2007. Vol. 450. Р. 1235—1239.
Romanov S. I., Pyshnyi D. V., Vandysheva N. V., et al. A silicon microchannel chip for biochip technologies // Nano- i mikrosistemnaya tekhnika. 2007. No. 9. P. 55—61 [in Russian].