Boron Neutron Capture Therapy for Cancer: At the Finish Line
Recent years witnessed a tremendous success in the treatment of oncological diseases yet they still remain one of the leading causes of death in developed countries, where life expectancy is on the rise. Many malignant brain tumors, such as glioblastomas, are still considered incurable – in Russia alone, about four thousand people die from them every year. The idea to irradiate tumor cells, saturated with the boron-10 isotope, with a stream of neutrons in a certain energy range emerged several decades ago. Outwardly simple, this technique of “cellular nuclear explosion” proved to be so hard to implement that in the entire world, not a single specialized complex for boron neutron capture therapy of cancer has so far come into existence. But scientists continue working. Through their effort at the Institute of Nuclear Physics in Novosibirsk, which has designed a compact new-type accelerating neutron source, Russia now has every chance of becoming a world leader in this promising therapy for the most aggressive cancers
The first neutron beam was obtained at our boron neutron capture therapy (BNCT) facility in 2008. Our main accomplishment of recent years has been not only to bring the facility into operation but also to gain an understanding of the processes that take place in it and make appropriate design modifications. This is important because we are dealing with a completely new type of a particle accelerator.
Today, a dozen different types of accelerators are in operation around the world, but none of them has yet obtained a high-current proton beam needed to bombard the target, i. e., a neutron source with specific energy characteristics. When designing our facility, we stayed off the beaten track, which appears to have been the right decision. In this field, there were experts much more experienced than us, but we must have had the amateur effect on our side – we came up with completely new, nonstandard ideas, which helped us to resolve seemingly unsolvable problems in designing compact BNCT facilities.
From Russia with an idea
All our discoveries and inventions have been patented; we have over a dozen Russian patents. One example of our inventions is the neutron-generating target. When we were only beginning to design our facility, a group of reputable scientists argued in their paper that a lithium target was the best one but no one could possibly create it. We now have such a target, and it has been working well for almost a decade.
Our story began almost two decades ago, when Grigory Silvestrov, head of laboratory at the Institute of Nuclear Physics SB RAS, received a call from his university coursemate who attended a conference in China on the prospects of boron neutron capture therapy (BNCT). Silvestrov often said that he was worth a lot as a physicist but he wished to do something tangibly beneficial for humanity. He got fascinated by the idea of creating an accelerating neutron source for BNCT and built a team of enthusiasts. Silvestrov himself died in 2003, when there was no “hardware” yet, only ideas, calculations and the first experiments on prototypes, but the work continued. As early as in 2007, the accelerator took on a visible shape, and the next year they obtained the first neutron beam
A remarkable event happened to me recently. I was invited to the Okinawa Institute of Science and Technology, an insanely funded Japanese analogue of the Russian Skolkovo. One of the tasks of the laboratory, headed by the former director of KEK (the famous Japanese accelerator complex), was to create a neutron-generating target. He had looked into the issue to conclude that creating such a target was impossible, but my friends and his colleagues from KEK convinced him otherwise. I said at their laboratory workshop that there was a very simple alternative idea of how to do it. I still remember the expression of shock on their faces… Now these targets are made all over the world; one usually cites our research when they publish their first paper, but then they forget about that. This is normal – in order to defend your ideas, you must not tread water but always come up with something new.
In October 2016, the city of Columbia, Missouri, became the venue for the Congress on Neutron Capture Therapy, which is held every two years. This event brings together chemists, biologists, and physicists, about 200 people in total. This time there were two representatives from Russia, myself and Vladimir Bregadze from the Nesmeyanov Institute of Organoelement Compounds (Moscow), who studies compounds for targeted delivery of boron. This fact shows what teams in our country have achieved real success in this field.
CELL EXPLOSION
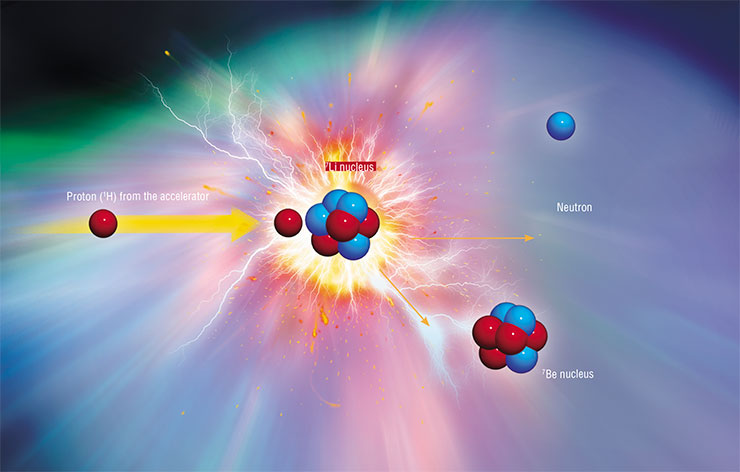
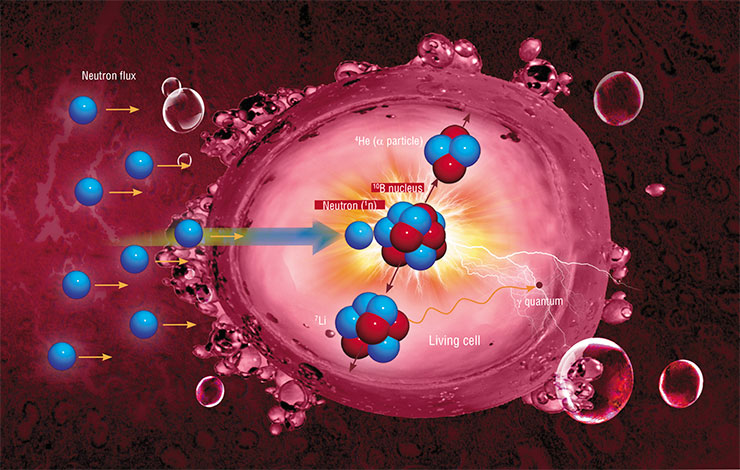
The idea of boron neutron capture therapy was first proposed in 1936, only four years after the discovery of the neutron. Its principle is to irradiate tumor cells, saturated with a stable boron‑10 isotope, with a flux of thermal neutrons. The boron‑10 nucleus is extremely effective in capturing a neutron even when it passes by at a distance one or two orders of magnitude greater than the nucleus itself. When the neutron is absorbed, two massive particles are formed. Due to the rapid deceleration of boron decay products, approximately 80 % of energy generated in this nuclear reaction is released inside the cancerous cell, leading to its irreversible destruction. The neutron flux must have a sufficient density, and the neutron absorption must reach a maximum at the tumor location depth. The so-called epithermal neutrons with energies from 0.5 eV to 10 keV meet these requirements best of all. The energy distribution should be as narrow as possible to minimize the contribution of the accompanying fluxes of slow and fast neutrons as well as gamma rays
I know only two more teams in the world who, as they say, have obtained a beam with the required parameters at accelerators of a different type, a joint Belgian – Japanese team and a US team, but only our results have so far been published. At the same congress, the Russian side came up with seven reports, three of which were presented by the Japanese. And a question was asked during the very first presentation: Why did they even go to Siberia? The answer was simple – Siberia has the only neutron source that actually works.
Among Russian specialists, I would also like to mention Vladimir Mitin, the head of the Veterinary Clinic at the Blokhin Cancer Research Centre, who successfully treated dogs using BNCT at the training nuclear reactor of the neighboring MEPhI. This work stopped after his death 11 years ago.
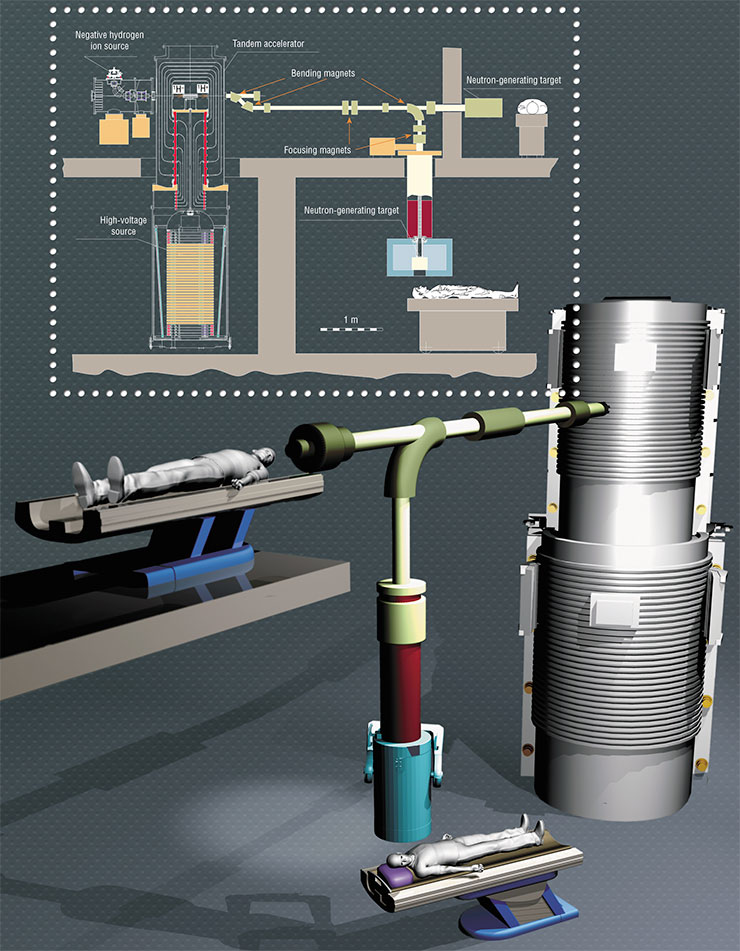
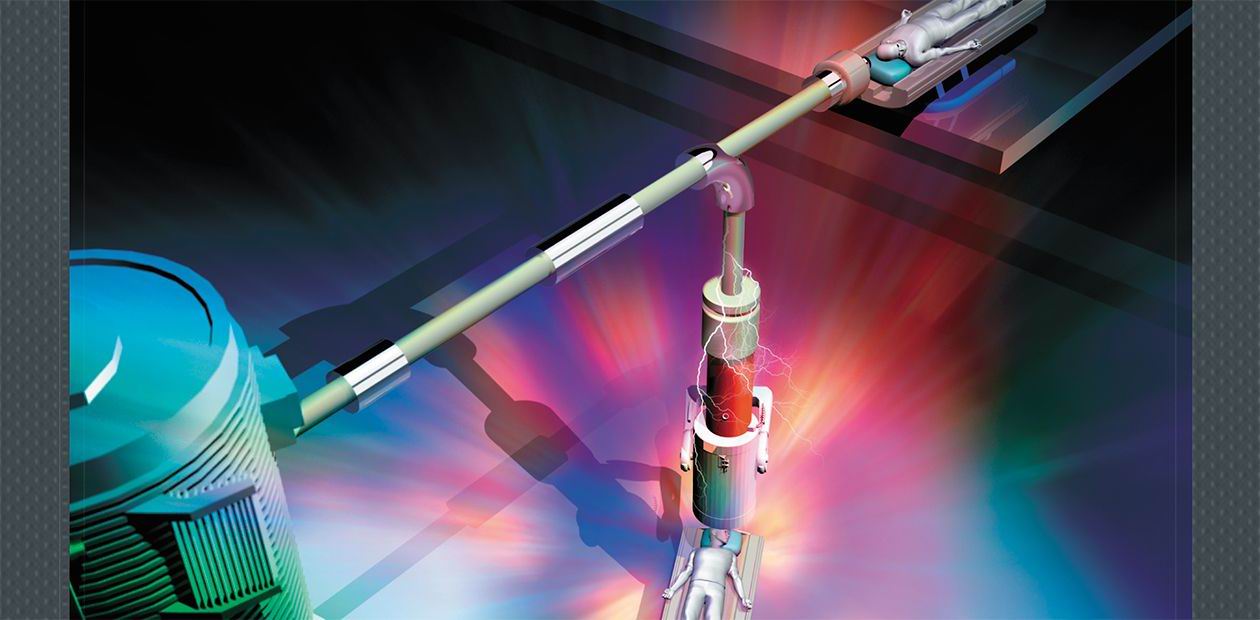
In conventional accelerators, charged particles are pumped with energy in an accelerating tube, which is essentially a series of metal rings, i. e., electrodes energized with a sequentially increasing potential. These rings alternate with dielectric ones, which get hit at a high current by secondary particles and ultraviolet radiation, possibly causing breakthroughs. This reduces the energy efficiency and stability of the facility.
In the new tandem accelerator, the standard accelerating tube is replaced by a cabbage-like structure with electrode “leafs” hanging on the isolator “stump” in a vacuum. A beam of negatively charged protons passes through the center of the “cabbage” at 90 ° to the “stump.” Since the electrodes are not separated by insulators, they can be placed closer to one another to yield a higher rate of particle acceleration
It should be noted that BNCT is quite expensive – drugs for targeted delivery of boron cost a lot of money because the demand for them is low given a sophisticated production process. Today, the drug dose required to treat one patient costs about 0.25 million rubles for borphenylalanine and for times as much for borcaptate. Fifteen years ago, however, these drugs would cost an order of magnitude more, and now every BNCT conference holds discussions around hundreds of new drugs for targeted delivery of boron. New ones every time though… Russia does not produce these compounds at all; we use drugs made by the Czech company Katchem.

From a global perspective, I believe physicists have done their job. Now we are modernizing our machine so that by the summer of 2017, regardless of funding, we could obtain a neutron beam suitable for treating patients, with a greater penetration depth, etc. This beam will be ideal in my understanding; I do not see how one can in principle make it better. This is why I added the letter ‘i’, meaning ‘ideal’, to our project title, iBCNT. Perhaps, it is still technically feasible to make some modifications, but it is impossible to improve the quality of the neutron beam at the current level of accelerating technology. It’s time to stop improving and start putting it to life!
Physicist have done their job
When we were planning our accelerator, we set the task of reaching a proton beam current of 10 mA, but at first we got only 1 % of that. The same story happened a year ago with our Japanese colleagues from the University of Tsukuba, who had got a similar machine from Mitsubishi but with a different type of accelerator – they planned to reach the same figure but got only 0.1 % of what they wanted.
My main dream back then was to reach at least 3 mA so that we could start treating people. And at the beginning of 2015, we got straight to 5 mA, having increased the current fiftyfold! In fact, today it is not the accelerator design that limits our possibility of further increasing the current. Moreover, in my opinion, these characteristics are more than enough for medical purposes. Nevertheless, next year we plan to reach the 10 mA threshold; this is now a matter of principle.

IDEAL TARGET
Exposure to a powerful proton beam leads to the heating of the irradiated material, and since the melting point of metallic lithium is only 182 °C, one requires very efficient heat removal. Initially, liquid gallium was used to cool the target, but later it was replaced by ordinary water. As a result, it was possible to find conditions enabling one to keep lithium in a solid state, thus limiting the spread of the radioactive beryllium‑7 isotope, which inevitably forms together with the neutrons.
Another Achilles’ heel of the lithium target is the associated parasitic gamma radiation. Efficient generation of epithermal neutrons occurs only in a very narrow surface layer of lithium; therefore, as protons move deeper, neutrons cease to be generated but gamma quanta are still emitted. It turned out that gamma radiation decreases substantially if further deceleration of protons occurs in a heavier metal than lithium. To this end, a thin (50–100 µm) layer of metallic lithium is applied to the substrate.
However, those protons that pass by the lithium layer undergo almost no scattering during deceleration and get stuck at virtually one and the same depth, where hydrogen accumulates over time. As gas pressure increases, the target surface begins to swell. In the course of experiments, researchers managed to find the most stable substrate material; in clinical use, it suffices to replace this target once a week
My next dream was to prove the effectiveness of our machine from the point of view of not only physics but also consumer value. To hit this mark, we had to work with cell cultures and laboratory animals. To solve the first problem, we bonded over with the Japanese University of Tsukuba, which has a well-known medical clinic. The only thing I will say about this clinic is that proton cancer therapy, which was introduced last year in the Protvino Naukograd near Moscow and proudly reported to Russian President Vladimir Putin, appeared in the price list of that clinic back in 1983, i. e., 33 years ago! And in 2001, the Japanese clinic replaced their proton facility with a more modern one.
In June 2015, I met with the clinic director Akiro Matsumura, who said, among other things, that Mitsubishi was unable to launch the BNCT accelerator. So we agreed to work together. This cooperation benefits both parties. We have the facility; the Japanese side has extensive experience in conducting research and, importantly, treating people with BNCT at the JRR‑4 reactor (Tokai), which was subsequently closed.

BORON BLASTER
Neutron capture therapy for cancer now uses compounds containing atomic nuclei of the stable (nonradioactive) boron‑10 isotope. The main requirements for these compounds are the ability to selectively accumulate boron in tumor cells, compared with healthy tissues; low toxicity; and solubility in water.
The most common choice today for BNCT is borcaptate (boric sulfhydryl) and borphenylalanine based on an aromatic amino acid. In recent years, researchers around the world have been vigorously looking for and synthesizing new potential boron-containing drugs. A wide variety of substances are being tested as boron carriers, both low- (derivatives of amino acids, i. e., precursors and analogues of nucleic acids; dipeptides; sugar derivatives, etc.) and high-molecular-weight compounds, such as antibodies and their fragments. The key task remains the same – to solve the problem of selective delivery of boron to tumor cells and accumulation of necessary amounts of boron in these cells (about 109 boron atoms per cell). Boron can be delivered to a tumor using nanoparticles in the form of liposomes, i. e., closed vesicles with water content and lipid walls. Boron-containing drugs can be incorporated both into the inner cavity of the liposomes and into their membrane (Shmal’ko et al., 2013). Recently, the INP SB RAS patented a method for delivering boron-containing drugs for BNCT into a tumor cell using modified liposomes, whereby a luminescent dye of one color is introduced into the lipid part of the liposome and a dye of a different color is added into its aqueous part. The drug delivery is controlled by comparing images obtained in the different colors (Taskaev et al., 2016)
For our research, four cell cultures were purchased from the Institute of Cytology, Russian Academy of Sciences (RAS, St. Petersburg), including two standard control cultures and two tumor ones, of human gliomas and glioblastomas. With the help of the Japanese colleagues and specialists from the Institute of Molecular and Cell Biology, Siberian Branch, Russian Academy of Sciences (Novosibirsk), series of experiments were carried out on irradiating these cell cultures. To accurately measure the boron concentrations in tissues and cells – with an eye to a possible future therapy – we acquired an expensive (about ten million rubles) Japanese mass pectrometer to measure the concentration of solutes. We financed it from a grant of the Russian Science Foundation, which is intended mainly for purchasing equipment.

Ultimately, we obtained an almost perfect result, when at a certain, sufficiently large, dose of radiation, the survival rate of healthy cells dropped by only 5 %, and that of cancerous ones, which accumulated boron, fell by 98 %! This result testifies to the high quality of the neutron beam, which consists mostly of “correct” epithermal neutrons, which are captured primarily by boron atoms.
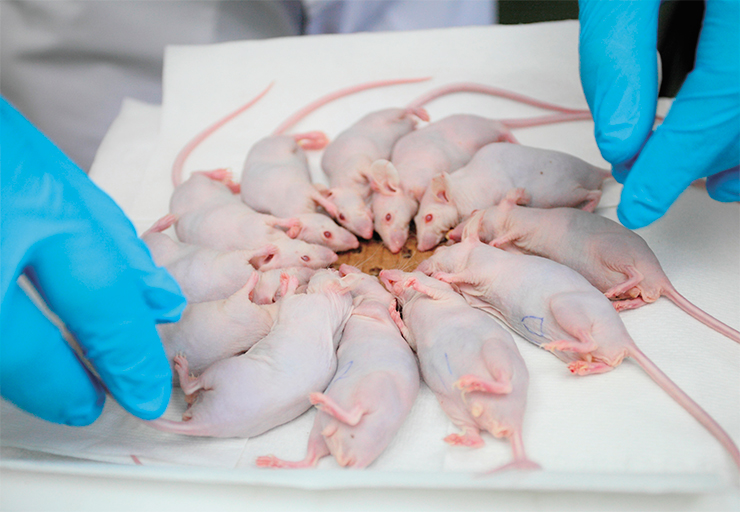
Simultaneously, we conducted, together with specialists from the Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences (SB RAS, Novosibirsk), animal trials on immunodeficient laboratory mice inoculated with a glioblastoma, i. e., a “human” brain tumor. Typically, these mice would die after about five weeks. We decided to irradiate them (albeit without much hope) five days before they were expected to die. For these experiments, special containers were made, with heating, where we placed the euthanized animals.

The cell culture studies use various lines of both healthy and tumor cells, mainly represented by brain tumors. The cells are incubated in a medium enriched with the boron‑10 isotope and then irradiated in the accelerator. The irradiation is followed by a clonogenic analysis to check the viability of the cells, specifically, their ability to divide and form new colonies.
Based on the irradiation experiments on animals, researchers have assessed the effects of BNCT, depending on the radiation dose. One of the most complicated tasks is to measure the dose received by the patient during BNCT because the total dose consists of several components – from the accompanying gamma quanta from the lithium target and the accelerator, from fast neutrons, from thermal neutrons, and from the boron neutron capture – and it is difficult to measure them separately. Each of these components depends on the lithium target geometry and the proton beam parameters. In addition, the dose from boron neutron capture also depends on the type, shape, and location of the tumor and the distribution of boron in it and in the surrounding tissues.
It is much more difficult to calculate all the four dose components and predict the effect on healthy and tumor tissues in BNCT than in traditional radiation therapy, where dose determinations usually rely on semi-empirical algorithms and measurements in a water phantom. Experiments on laboratory animals with known dose – response curves are one of the options for developing an empirical therapy planning algorithm; i. e., the biological consequences of irradiation in animal tissues may help to more accurately determine the dose, compared with calculations.
All animals without a tumor remained alive after irradiation; no signs of pathological effects on healthy tissues were found. Currently, cellular effects of irradiation are studied in more depth, including the processes of hematopoiesis, the state of tissues of vital organs, and the effect of boron-containing drugs on the animal’s body, in order to find the optimal dose.
After the final trials of the technology on animals, it is planned to move on to clinical trials, i. e., therapy on cancer patients.
In the most successful trial, the tumor completely resolved in three out of five animals, which was confirmed by tomography, and the animals became healthy – they lived in the vivarium for another two months and were euthanized only because the experiment was over. This is a truly fantastic result. After all, borphenylalanine, which we inject, accumulates not only in the tumor but also in the liver and kidneys. Moreover, the mouse is a small animal so, unlike humans, its whole body is irradiated, causing more damage to healthy tissues. One should also bear in mind that in the late stages of tumor development, the death of cancer cells may cause large-scale necrosis. But even under all these aggravating circumstances, we were able to cure the animals.
Time to put BNCT to life
In Russia today, BNCT is a new promising and, most importantly, proven method of treating cancer tumors, so it would be absurd for the government and big business not to support it. Japan is now developing five successful BNCT projects using different types of accelerators within public–private cooperation agreements with industrial giants such as Mitsubishi and Toshiba. In fact, these are all government investments.
The shortest and easiest way for us to obtain funding for the construction of a BNCT facility is to receive support within a breakthrough scientific project based on Strategic Academic Units of Novosibirsk State University (NSU). According to the project, within a very short period (cofounding is provided for four years), we are to build a facility for direct medical use and treat at least 10 patients. This is an ambitious plan, not so easy to implement, but we are used to setting goals that at first glance appear impossible. In fact, preparatory work for the implementation of this project has already begun.
FROM A LECTURE by Prof. VLADIMIR BREGADZE, Dr. Sci. (Chem.) (Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, Moscow)
We need to think proactively and search for compounds that will accumulate in tumors more selectively. The main principle of their production is to create molecular conjugates from a boron-containing component and a part responsible for targeted delivery to tumor cells. In so doing, the distance between the components is the key because the active part of the conjugate may negatively affect the properties of the “vehicle.”
The priority candidates for the transport molecule are amino acids as well as other low-molecular-weight compounds (porphyrins, nucleotides and nucleosides, lipoproteins, etc.). All these compounds are welcomed by tumor cells, which have an affinity for them, and hence they can accumulate in the cells.
We have obtained conjugates of derivatives of polyhedral boron compounds with the various porphyrins, specifically with chlorin E 6. We hand over all the synthesized compounds for testing to the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry (Moscow) to assess their accumulation in tumors and healthy tissues. Studies have shown that the intensity of accumulation of the boron-containing conjugate in lung carcinoma cells is indeed higher in the case of a greater distance between the boron part and the chlorin E 6 molecule.
The maximum ratio of drug concentrations in tumor and healthy tissues, which we have achieved to date, is 5:1. This is better than for the currently applied borphenylalanine (3:1), but we should be striving for more
The project also plans to develop an improved drug for targeted delivery of boron, a Russian analogue of borphenylalanine. This will be done by the Institute of Organic Chemistry SB RAS (Novosibirsk), which has its own pilot production, together with the Nesmeyanov Institute of Organoelement Compounds RAS (Moscow).
It is known that NSU plans to submit an application for the second phase of construction, which will include a clinic for practicing BNCT. But this is a matter of uncertain future, so the current project application also includes another scenario. According to the backup plan, the Institute of Nuclear Physics modernizes one of its buildings to locate the accelerator as well as space for receiving and treating patients. The throughput capacity of this facility will be sufficient because the BNCT procedure lasts one hour and is usually done once.
While we were working on our facility and searching for ideal solutions, we had to resolve, along the way, many problems that went far beyond the scope of accelerator physics. Together with neurosurgeon Dr. Vladimir Kanygin from the Dorozhnaya Hospital (a part of the healthcare network of Russian Railways), we have developed and patented a system for generating a correct neutron beam, which allows one to change the beam’s direction. We managed to find a simple engineering solution that not only allowed us to irradiate the patient from any side but also improve the beam quality.
In conversations with our Japanese colleagues, an absolutely new idea emerged to develop BNCT dosimetry, and this invention has also been patented by now. Once I went skiing with Andrei Sokolov, a fellow research from my institute, and our conversation resulted in a patent for the generation of monochromatic neutrons to search for dark matter. Now, together with Alexander Shmakov from the Institute of Catalysis SB RAS and Sergey Gromilov from the Institute of Inorganic Chemistry SB RAS, we are discussing the possibility of adapting our machine for neutron diffractometry to determine the structure of light elements in a substance.
All the ideas that we are patenting are, in fact, very trivial, but for some reason they come only to those not burdened by experience, i. e., to amateurs. As I said above, this is how we created our facility. Being initially a specialist in plasma rather than accelerators, I still wonder (to be honest) how we managed to accomplish all that.
Over the past five years, we have turned from specialists, potentially attractive for BNCT, into real players in this field. Over the last year, we have actually worked on consumer value. This is crucial because physicists often think: Well, we did a great thing but why aren’t users rushing in and grabbing it? This thinking comes to nothing. One has to make real effort so that the work on implementing one’s ideas and inventions could give concrete results.

A COMMENTARY SIX YEARS AFTER:
As predicted six years ago, boron neutron capture therapy (BNCT) did not turn away from the finish line and became a working treatment method 84 years after it was proposed. The first two BNCT clinics have been launched in Japan and another four have been built, of which the clinic in China is equipped with our neutron source. An even greater number of projects are currently underway, including the manufacture of a neutron source for the Blokhin Cancer Research Centre in Moscow. For us as a research team, this finish line turned out not to be straight – it forked. We are being sought after as developers for many other applications as we have learned how to generate a zoo of particles: neutrons of any energy, photons, alpha particles, and positrons. In addition to developing dosimetry methods and testing targeted boron delivery drugs for BNCT, we measure nuclear reaction cross-sections, test materials for the ITER fusion reactor, prepare to test optical cables for the CERN Large Hadron Collider, and much more
To date, about two thousand people worldwide have been treated with BNCT, using the existing scientific and educational nuclear reactors, many of which are no longer in operation. It is needless to explain the importance of this therapy as it works on tumors that are currently considered incurable, such as glioblastomas and cancer metastases. However, this method is still viewed, in fact, as experimental, and specialists will have to make effort to develop adequate treatment strategies and protocols.
Today, our facility is the world’s only operational compact source of neutron beams suitable for BNCT. We are now ready to come up with an operational medical accelerator, which will allow the treatment of patients in short run. However, both of our financial support grants – from the Ministry of Education and Science of Russia for the improvement of the facility and from the Russian Science Foundation for biological research – are ending in December this year. What will tomorrow bring?
References
Neutron Capture Therapy: Principles and Applications. Eds.: W. Sauerwein, A. Wittig, R. Moss, Y. Nakagawa. Springer, 2012. 533 pp.
Taskaev S. Yu and Kanygin V. V. Bor-neitronzakhvatnaya terapiya (Boron Neutron Capture Therapy). Novosibirsk: Sib. Otd. Russ. Akad. Nauk, 2016. 216 pp. [in Russian].
This publication uses photographs by Alexander Makarov