VITA Means Life. Boron Neutron Capture Therapy of Cancer
Oncological diseases are among the most common causes of human death, despite all successes achieved in fighting them. The idea of boron neutron capture therapy of cancer, which has turned out to be effective in curing previously incurable tumors, appeared 75 years ago, but so far no operating specialized system based on this method has been developed. Specialists from the Budker Institute of Nuclear Physics of the Siberian Branch of the Russian Academy of Sciences (BINP SB RAS), who became involved in solving this problem approximately a decade ago, have succeeded in creating an operating prototype of a facility that can be used in oncological hospitals
The idea of selective affection of malignant tumor cells by the method of boron neutron capture therapy (BNCT) was first proposed in 1936, only four years after the neutron was discovered.
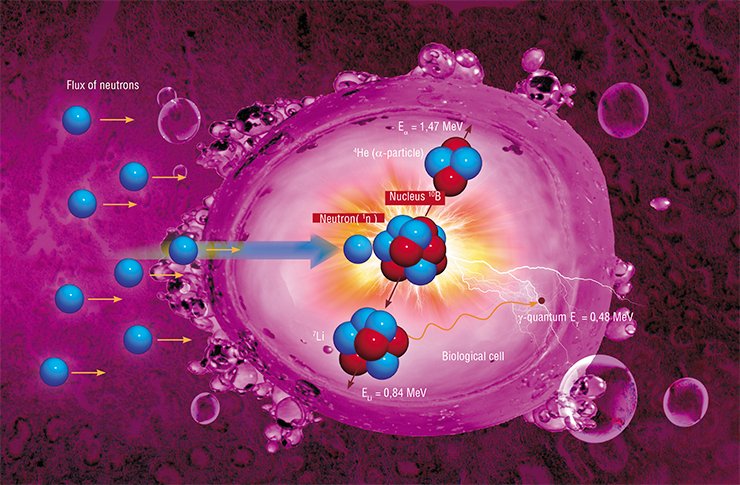
Its physical principle is simple and elegant. A stable boron isotope 10B is injected into the tumor, which is subsequently irradiated by a flux of neutrons. When the neutron is absorbed by the boron-10 nucleus, two massive particles are formed, which impart their energy during deceleration at a micrometer distance, i.e., within the limits of the cell “marked” by boron, resulting in an irreversible destruction of this cell.
The beauty of this method is due to several gifts of nature. First, the boron-10 nucleus is extremely effective in capturing the neutron: the cross-section of thermal neutron absorption is 4•10 –25 m2, which is thousands times greater than that ensured by nuclei of other elements. The second favorable feature of boron is rapid deceleration of the products of its decay, which ensures locality of the process: approximately 80 % of energy generated in the nuclear reaction is released inside the cancerous cell proper. Finally, the most important advantage of boron is the fact that it forms compounds that are not toxic for the human organism, such as boronophenylalanine (BPA) or boron sulfhydryl (BSH), which are usually used as contrasting media for diagnosing tumors with a magnetic resonance tomography, but can also be used for targeted delivery of boron to the tumor.
Despite its apparent simplicity, BNCT implementation is rather difficult. Its history includes ups and downs, and now it is considered as an art, which will possibly allow fighting against previously incurable tumors in the near future.
Curing the incurable
The first biological BNCT experiments on mice were started during the World War II. In 1951, researchers demonstrated for the first time that the use of boron compounds with some aminoacids ensured a higher concentration of this element in malignant cells than in healthy cells. Indeed, malignant cells are prone to enhanced reproduction, which is accompanied by intense synthesis of protein; therefore, they need more aminoacids. When a solution of a boron-containing aminoacid was injected into patient’s blood, boron was predominantly concentrated in tumor cells within several minutes.
By the late 1950s, special nuclear reactors for the first clinical tests were constructed in Brookhaven and Massachusetts (USA). Unfortunately, the expected therapeutic efficiency was not reached, and the experiments were terminated. As it was found later, the reason for this failure was an insufficient concentration of boron in cells. The point is that neutrons interact not only with boron, but also with nitrogen and hydrogen nuclei, which form more than 70 % of biological tissues. Because of the low boron concentration, the main portion of radiation absorbed by the patient was non-selective “background” radiation induced by this interaction.
Despite the failure, one participant of these experiments, a Japanese neurosurgeon Hatanaka, continued to practice BNCT when he returned to Japan in 1968. He performed craniotomy directly on the premises of a nuclear reactor, removed the tumor from the open brain, and then irradiated the operated area by a beam of thermal neutrons with prior injection of boron-containing agents into the patient’s organism. In Japan, more than 200 patients were successfully treated in this manner. Hatanaka’s first patient with brain glioblastoma stayed alive for 21 years instead of half a year, which is a usual period for such a diagnosis. Owing to these results and also to the progress in synthesis of effective agents for boron delivery to tumor cells, since the 1990s clinical tests of the new method have been regularly performed in many countries.
The most important advantage of this method is its orientation to treatment of malignant tumors that can hardly be cured by therapy, including brain glioblastomas and melanoma metastases. You can judge by yourself: brain glioblastoma is diagnosed with one person per 20,000 people, and the outcome is always fatal: surgery and conventional radiotherapy cannot prevent propagation of the tumor over the brain; these methods can only prolong the patient’s life by no more than for a year
Encouraging results were also obtained in the field of neutron capture therapy of liver, which is temporarily extracted from the patient’s body for irradiation. Today, researchers consider a possibility of using the new method for treating the cancer of the oral cavity and thyroid gland, as well as rheumatoid arthritis, which is an extremely serious and yet incurable non-malignant autoimmune disease.
Competition for the role of a target
The atomic reactor is not the most convenient tool for testing medical methods; moreover, it is absolutely inconvenient to use the atomic reactor in oncological hospitals. Less expensive and more compact sources of neutrons are needed.
In 1994, the first international workshop on “accelerating” the sources of neutrons for BNCT was held, where many projects were considered and requirements to technical characteristics of accelerators were formulated. In practical implementation of these projects, however, specialists faced a number of problems.
The point is that irradiation by a flux of thermal neutrons should be performed one time, and its duration should be no more than an hour; this regime of therapy is caused by a high cost of boron-containing drugs and by specific features of the kinetics of boron accumulation in internals. This means that the neutron flux should have a sufficient density, and the maximum absorption should be reached at the depth where the tumor is located. The best option is to have neutrons with energies from 0.5 eV to 10 keV. It is necessary to form a neutron flux with the narrowest possible distribution in terms of energy to ensure minor contributions of the accompanying fluxes of thermal neutrons, fast neutrons, and gamma rays to the dose absorbed by the patient. It was rather difficult, however, to obtain such “intermediate” epithermal neutrons.
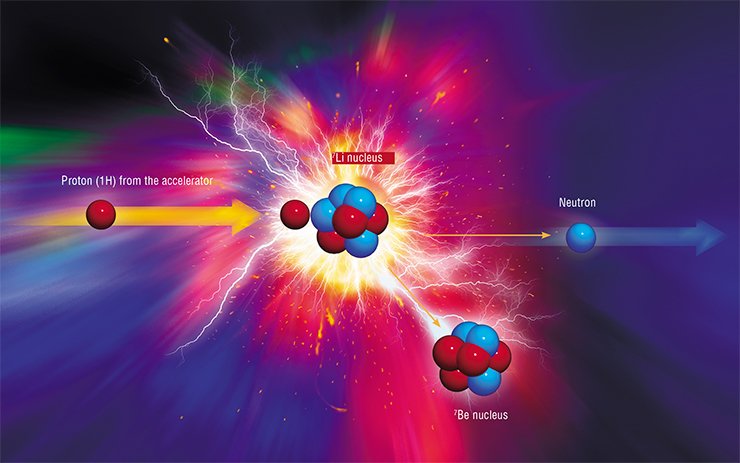
The best method of generation of epithermal neutrons is commonly assumed to be bombardment by lithium nucleus protons: the flux of “knocked out” neutrons is sufficiently dense, and their energy spectrum is comparatively soft. Nevertheless, researchers believed ten years ago that it is next to impossible to make a target of metallic lithium because of its softness, low melting point, weak thermal conductivity, and high chemical activity. Alternative options are beryllium-9 and carbon-13. It is simpler to make targets of these materials, but the beams of charged particles for their bombardment should be much more powerful.
Researchers from BINP SB RAS (Novosibirsk) became interested in BNCT implementation at the end of the 1990s. At that time, potentially suitable accelerators provided currents of beams of charged particles equal to several milliamperes only, whereas the required value was at least 10 mA for the lithium target and more than 30 mA for other targets. Novosibirsk physicists decided to follow an unbeaten track.
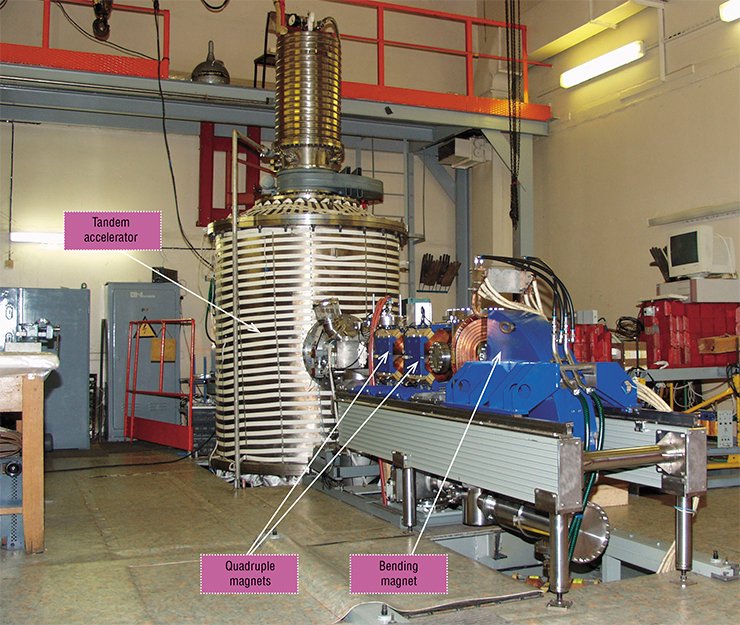
VITAl accelerator
The idea was to develop an accelerator of a new type. In conventional accelerators, energy is pumped into charged particles in an accelerating tube. It is formed by metallic rings-electrodes with an increasing potential fed to them. The electrodes are insulated from each other by dielectric rings; as the current increases, however, secondary particles and ultraviolet radiation from beam passage in the residual gas are incident onto the electrodes. This leads to insulation breakdowns, which decreases the energy efficiency and stability of device operation.
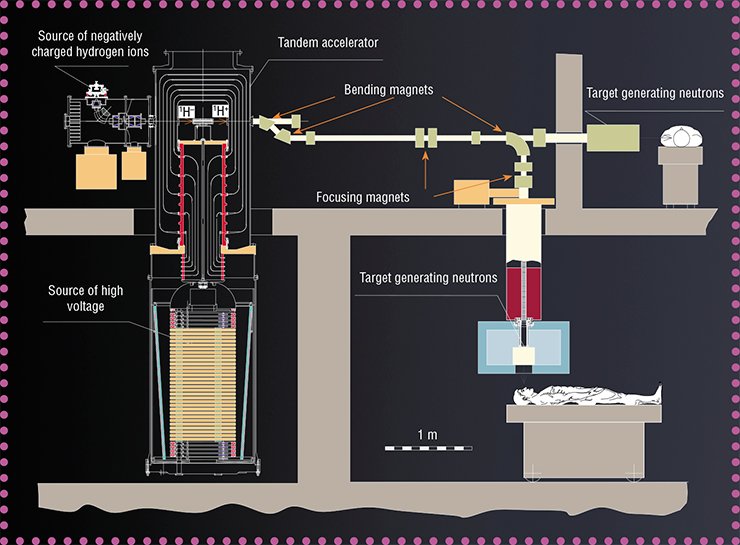
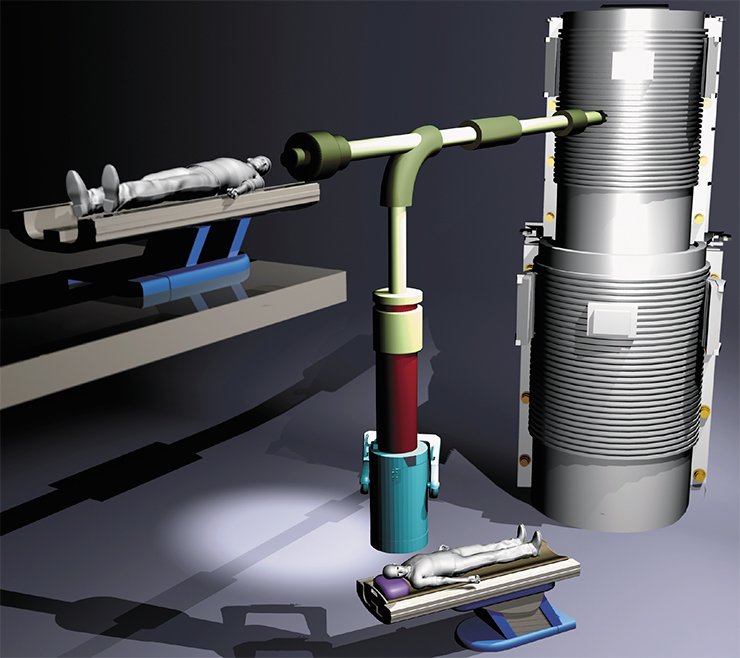
The main idea was to ensure the greatest possible distance between the insulator and the particle beam. The developers of the new accelerator decided to cancel the standard accelerating tube because they expected the unconventional structure to generate a greater current. Figuratively, the new accelerator can be presented as a head of a cabbage, where “leaves” (electrodes) are attached to the “cabbage stalk” (insulator) in vacuum, and the particle beam passes through the “cabbage head” center at an angle of 90° to the “cabbage stalk.” Here the electrodes are not separated by insulators; therefore, they can be located closer to each other, which ensures high acceleration of particles.
The expenses of the novel structure of the accelerator were large-size electrodes and, as a consequence, large energy stored between them. This means that inevitable breakdowns could irreversibly spoil the electrode surface. Though exact data on this issue were not available at that time, the scheme feasibility was questionable. Fortunately, the apprehension was dispelled, and a proton beam with a record-beating current of 3 mA was obtained already in the first experiments on a real device designed at the laboratory by 2007.
In the same year, the Ministry of Education and Science appreciated the new accelerator aws the next (third) unique facility developed at BINP SB RAS. The potential of this facility can be deduced from its name VITA (Vacuum Insulation Tandem Accelerator).
Nonstandard target
The next unusual decision was to make the target from the “unsuitable” lithium. The developers of the new accelerator managed to solve two main problems, which used to make this element unsuitable for target fabrication.
 The first problem is the fact that the action of a powerful proton beam leads to heating of the irradiated material. The melting point of metallic lithium is only 182 °С; therefore, the use of this element requires extremely effective heat removal. Initially, the target was cooled by liquid gallium, but then usual water turned out to be good enough. As a result, it became possible to choose conditions that allowed lithium to be retained in a solid state, whereas the target was heated by the proton beam with a power up to 0.5 kW/cm2. Moreover, this procedure allowed significant restriction of propagation of the beryllium-7 radioactive isotope, which is inevitably formed together with neutrons.
The first problem is the fact that the action of a powerful proton beam leads to heating of the irradiated material. The melting point of metallic lithium is only 182 °С; therefore, the use of this element requires extremely effective heat removal. Initially, the target was cooled by liquid gallium, but then usual water turned out to be good enough. As a result, it became possible to choose conditions that allowed lithium to be retained in a solid state, whereas the target was heated by the proton beam with a power up to 0.5 kW/cm2. Moreover, this procedure allowed significant restriction of propagation of the beryllium-7 radioactive isotope, which is inevitably formed together with neutrons.
Another Achilles’ heel of the lithium target was the accompanying spurious gamma radiation induced during deceleration of the proton beam. Effective generation of epithermal neutrons occurs in an extremely narrow surface layer of lithium (within several micrometers); as soon as the energy of protons decreases below 1.882 MeV, neutrons are no longer generated, though gamma quanta are still emitted.
And yet, there are no situations without a way out. It turned out that the level of gamma radiation can be substantially reduced if further deceleration of protons occurs in a heavier metal rather than in lithium. For this purpose, an extremely thin (5—100 µm) layer of metallic lithium is deposited onto a substrate with the use of advanced technologies.
This solution, however, generated a new problem associated with radiative damages of the target substrate. Protons that “slipped” through the lithium layer are practically not scattered during deceleration and, therefore, stick in the substrate approximately at the same depth. With time, they become accumulated there in the form of hydrogen. The gas pressure increases, and the target surface starts to swell out. Though this process is unavoidable, researchers managed to choose the maximum stable material of the substrate. Such a target is fairly durable: it is sufficient to change it once a week in the hospital.
Nevertheless, this is not the end of the story. Generation of neutrons is inevitably accompanied by accumulation of radioactive beryllium-7 inside the lithium layer. The half-life period of beryllium-7 is 53 days. Therefore, a special protective buried container had to be designed for temporal storage and deactivation of the radioactive target. As a result, long-time experiments became possible.
Eventually, the researchers eliminated all drawbacks inherent in the lithium target and in 2008 performed the first generation of neutrons.

Тoday, researchers have managed to solve all physical problems that had prevented creating a compact accelerating source for neutron capture therapy of cancer. Thus, in less than a decade, BINP SB RAS specialists have developed and fabricated an operating prototype of the facility: a compact and comparatively inexpensive source of neutrons, specially designed to be used in oncological centers.
Meanwhile, the BNCT method proper is not yet ready for wide applications in hospitals: it requires further joint studies of physicists, doctors, biologists, and chemists. A team of young researchers, postgraduate and undergraduate students are now working on this facility. They are supported by specialists from various research and educational centers of Novosibirsk. The first biological experiments were performed last year, and continuous generation of neutrons was maintained for several hours.
At the moment, the Novosibirsk BNCT facility is the best in the world. In terms of time, our researchers are rather close to reaching the goal. Theoretically, the project can be completed within two to three years. Is it a long or a short period? It depends on the person involved: the researcher performing tests, the official responsible for the project, and the patient for whom this new method may be the last hope will give different answers.
Nevertheless, all parties are interested to have the project completed, that is, the accelerator implemented into clinical practice, as soon as possible. Another party benefiting from it is the state, which will no longer lose a large number of citizens every year and will get a chance to become a leader in this vitally important field. What can be done to speed up the process? The development and implementation of high-tech methods of medical treatment in all countries, in particular, in Japan, where a similar center is under construction, are financed by the government. For the new facility developed by Siberian researchers, governmental investments are a matter of life and death, as well as for thousands of sick people.
References
Locher G. Biological Effects and Therapeutic Possibilities of Neutrons // Am. J. Roentgenol. Radium Ther. 1936. V. 36. P. 1—13.