The Life of a Chemist
This article is dedicated to a great Russian scientist, an outstanding chemist Vladimir Nikolaevich Ipatieff, the founder of the science and practice of heterogeneous catalysis at high temperatures and pressures, whose 150th birthday anniversary was celebrated on November 21, 2017. It was about him that Richard Willstätter, winner of the 1915 Nobel Prize in chemistry, said in 1942 that never in the history of chemistry had there been a greater man than Ipatieff. The life path of this great scientist is described both in his own writings and in those by his students and researchers of his creative work.
In some countries, for example, in the United States, it is believed that Russia produced three outstanding chemists: Lomonosov, Mendeleev, and Ipatieff. In Russia, however, the name of the latter received the recognition it deserved only in the last decades. In 2011, the Kalvis publishing house printed, in two volumes, the first Russian reedition of Ipatieff’s book The Life of a Chemist. Memoirs (New York, 1945); another autobiographical volume dedicated to his life in the United States in 1930–1941 (Ipatieff, 1959), as well as some other works, will soon be published. Since the milestones of Ipatieff’s life path are well detailed in the literature, this article focuses on the creative work of this remarkable scientist, who saw his duty in serving the people rather than those in power
You, Russians, cannot even comprehend who Vladimir Ipatieff was.
Every hour of his life here, in the United States,
every step in his research, he dedicated it all to Russia.
His limitless love for his motherland, which I have never seen in any of the emigrants,
was the soil on which grew the outstanding results of his scientific work...
Herman Pines, chemist; student, friend,
and the executor of the will of Vladimir N. Ipatieff, 1967
 Vladimir Ipatieff became fascinated by chemistry as early as in the sixth grade of the gymnasium. A chapter on chemistry he read in a textbook of physics fired his imagination. For the first time, he looked at the world with his eyes open and felt a craving for study in order to understand it better, as he would write later in his memoirs (Ipatieff, 1945). After the gymnasium, he went to military schools, where chemistry was given little attention, but he continued to study it on his own by reading textbooks and doing experiments in a small private laboratory where he reproduced all the problems from the analytical chemistry course by Prof. N. A. Menshutkin (1865—1902). At the Mikhail Artillery Academy, he was fortunate to attend the chemistry course taught by Alexei E. Favorsky (1860—1945), a student of A. M. Butlerov and a future well-known organic chemist and academician (1929). It was Favorsky who consolidated him in his wholehearted love of chemistry (especially organic chemistry) and thereby determined his fate. Upon graduation from the academy, Ipatieff was promoted to captain of artillery and got a position of a chemistry instructor at the academy. In the same year, he married an old friend, Varvara Ermakova. They went together through all the vicissitudes of fate up to the end; she passed away only nine days after her husband.
Vladimir Ipatieff became fascinated by chemistry as early as in the sixth grade of the gymnasium. A chapter on chemistry he read in a textbook of physics fired his imagination. For the first time, he looked at the world with his eyes open and felt a craving for study in order to understand it better, as he would write later in his memoirs (Ipatieff, 1945). After the gymnasium, he went to military schools, where chemistry was given little attention, but he continued to study it on his own by reading textbooks and doing experiments in a small private laboratory where he reproduced all the problems from the analytical chemistry course by Prof. N. A. Menshutkin (1865—1902). At the Mikhail Artillery Academy, he was fortunate to attend the chemistry course taught by Alexei E. Favorsky (1860—1945), a student of A. M. Butlerov and a future well-known organic chemist and academician (1929). It was Favorsky who consolidated him in his wholehearted love of chemistry (especially organic chemistry) and thereby determined his fate. Upon graduation from the academy, Ipatieff was promoted to captain of artillery and got a position of a chemistry instructor at the academy. In the same year, he married an old friend, Varvara Ermakova. They went together through all the vicissitudes of fate up to the end; she passed away only nine days after her husband.
Early years
There is evidence tracing the noble family of Ipatiev back to the times of Ivan the Terrible, or even further. The name of Ipatiev derives from Saint Hypatius (‘the highest or the best’ in Ancient Greek), the Archpriest of Gangra, who was tortured and murdered by heretics in the year 326 on the territory of modern Turkey. The Ipatiev Monastery (also called Hypatian Monastery) of the Russian city of Kostroma was erected in 1330 in honor of this saint. This monastery played a prominent role in the events of the Time of Troubles. On March 14, 1613, it hosted a solemn ceremony whereby the 16-year-old Mikhail Fedorovich Romanov was announced Russian Tsar, which marked the beginning of the reign of the Romanov dynasty in Russia.In some inexplicably way, the fate of the Romanovs intertwined closely with that of the Ipatievs. The Ipatiev Monastery became the cradle of the Romanov dynasty; Ipatiev House, which at that time belonged to the engineer Nikolai N. Ipatiev, a younger brother of Vladimir N. Ipatieff, became the last home of the last tsar of the Romanov dynasty, Nicholas II, who was shot there together with his family and heir on the night of July 17, 1918. However, these questions go beyond the scope of this article, which aims to describe Ipatieff’s career in science
The second teacher who deeply influenced Ipatieff was the famous Adolf von Baeyer (1835—1917), the future Nobel Prize winner (1905). Ipatieff worked at his laboratory in 1896—1897 at the University of Munich, during his one-and-a-half-year scientific internship, granted to the most promising young instructors of the Mikhail Academy. Baeyer took Ipatieff only at the express request of his old acquaintances from Russia. In Munich, Ipatieff investigated the structure of carone (the bicyclic ketone C10H10O) and completed his determination of the structure of isoprene (2-methyl‑1,3-butadiene), which he had started in St. Petersburg, and, for the first time, carried out its synthesis (until then, isoprene was obtained only by depolymerization of natural rubber, which consists of its monomeric units). When the work was done, Baeyer suggested that they publish papers under joint authorship, an offer he would seldom make (he usually would not cite a young researcher as a coauthor). The internship at Baeyer’s proved extremely useful for Ipatieff because there he mastered the most advanced methodology of chemical experiment and analysis of that time. Ipatieff took a full course of organic chemistry offered by Bayer and several elective courses; he benefited greatly from the almost daily conversations with Baeyer, who made a round of the laboratory at the beginning of each day, discussing results and correcting plans (Ibid.).

My brother and I took our religion so seriously that, when I was twelve, if we had been too lazy to study our lessons, we often got up early and stopped at church on the way to school in order to pray that the teacher would not call on us. Our mother would have explained how foolish this was, and we might have worked harder thereafter. But without her we had no one with whom to discuss such matters. The teacher of religion, the Reverend Mr. Sakharov, was a man of high religious education but could not gain the respect of the students or hold their interest. His attitude toward his work was purely formal and had no effect on his students’ lives.
My mother died of consumption shortly after her return from the Crimea, when I was twelve years old and in the third class at the Gymnasium. Anna Vasilievna Mazanova continued her position as mistress of the house, and ten months later became our stepmother… …My stepmother was the exact opposite of my mother, who was a charming and delightful hostess, with the ability to surround herself with interesting people. During my mother’s life, as I was told by my aunt, our house was often visited by educators, doctors, and other members of the intelligentsia.
…At the age of fourteen and a half, still a mediocre student, I was promoted to the sixth class. Soon after this promotion I began to be interested in my work and to study hard. My favorite subject was mathematics, which I studied beyond the class requirements under Uncle Mitia. Thereafter my report card showed a steady improvement, especially in science courses. It was much more difficult to obtain high grades in the Third Cadet Corps than in the other corps, especially those located in provincial cities. Our teacher in mathematics, Professor Protopopov, was very miserly with high grades, and in spite of my perfect answers I never received a grade above ten, which was the highest that he ever gave.
Protopopov, nevertheless, believed that I had sufficient ability to study higher mathematics and that I should be admitted to an artillery or engineering school. Upon graduating from the Corps I applied for admission to the Mikhail Artillery School; but I knew when I did so that my mathematics grades would ruin my chances, since only fifty or sixty boys were admitted from the twenty Cadet Corps. I was rejected and had to enter the Alexander Military School located in Moscow.
On August 31, 1884, in my sixteenth year, I left my father’s house forever, thus releasing him from further expense for my education, because the military school supported its cadets on a government grant. My entire education had cost my father almost nothing. During my seven years’ schooling in the Gymnasium the cost was no more than 350 rubles, plus clothes and living expenses at home. As I said goodbye to my father he told me that he would give me three rubles a month for gloves and tobacco and added: “In your life be faithful to God, be truthful and honest, and beware of women.” I was now independent, except that my freedom was severely limited by the military discipline of the School. Adapted from: (Ipatieff V. N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
Ipatieff presented his dissertation “The Action of Bromine on Tertiary Alcohols and of Hydrogen Bromide upon Acetylene and Allene Hydrocarbons in Acetic Acid Solutions,” the topic of which was suggested by Favorsky, at the Mikhail Artillery Academy in 1895. The dissertation was accepted, and he became a lecturer on staff of the academy. The Russian Physicochemical Society (RPCS; it functioned as a department of the Academy of Sciences) awarded Ipatieff for this work with the Small Butlerov Prize. In 1899, he was appointed Professor Extraordinary and in 1902, Professor Ordinary of Chemistry; since 1909, he had been the head of chemical laboratory at the Artillery Academy, teaching courses in theoretical and inorganic chemistry and giving practical classes in analytical chemistry. When preparing for his lectures, he took notes, which he later used to write his first textbook Inorganic Chemistry, which was reprinted several times (Ipatieff and Sapozhnikov, 1920).
Ipatieff’s bomb
I was made an officer on the 7th of August, 1887, a day memorable for the solar eclipse when the famous chemist, Mendeleev, made a scientific flight in a balloon.On becoming an officer each appointee received a sum of money from the government for a saddle and other equipment; but as this would not pay for everything, my father gave me some extra cash for clothing. After making my purchases I found that I still had one hundred rubles left. I could not then decide whether to buy a winter coat or a small chemistry laboratory which I had always wanted. I did not hesitate long, and of course the laboratory was my choice. I rationalized that a regular officer’s overcoat was much warmer than the soldier’s coat I had been wearing as a cadet, and I never regretted my choice, for with the small laboratory I experimented at my leisure and acquired a fundamental knowledge of inorganic chemistry. Adapted from: (Ipatieff V.N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
In 1897, Ipatieff became the first to synthesize isoprene from ethanol and thereby laid the groundwork for the synthesis of synthetic rubbers, resins, and gums. In 1900, he began his studies in the field of heterogeneous catalysis. At a RPCS meeting in January 1901, he made a detailed presentation on catalytic decomposition of alcohols. In the same year, Ipatieff’s report “On the Double Catalytic Decomposition of Alcohols” became the center of attention at the seminar of the German Chemical Society and at the 10th Congress of Russian Naturalists and Physicians (Ipatieff, 1945). In subsequent years, his research focused on catalysis at high temperatures and pressures in hydrogen medium. In 1904, he invented the famous “Ipatieff’s bomb,” a failsafe autoclave made of artillery steel for studies at pressures of up to 1000 atm. He took the sealing idea from artillery and pioneered the studies of catalytic transformations of organic molecules at high temperatures (up to 700 °C) and pressures (up to 1000 atm). Before Ipatieff, everyone believed that studies at elevated pressures and high temperatures were too complicated and dangerous. Moreover, organic chemists followed the advice of Butlerov, who wrote that the correctness of conclusions about the molecular structure of substances “…should best be evaluated by investigating the ways of their synthetic formation, preferably such syntheses that occur at a slightly elevated temperature and, generally, under conditions where one can track the gradual complication of a chemical particle” (Butlerov, 1953). Therefore, the principle of observing “mild conditions” of chemical reactions lay at the heart of all the experimental works conducted by Butlerov and his school, as well as most representatives of the classical trend in organic chemistry. Ipatieff dared to go against the advice of the great Butlerov and discovered a new continent in the world of molecule transformations in organic chemistry and petrochemistry.

In 1909, Ipatieff showed for the first time the fundamental possibility of obtaining butadiene (divinyl) from ethyl alcohol on an aluminum oxide catalyst, and in 1913, he was the first to synthesize polyethylene. Previously, Butlerov had attempted to carry out the polymerization of ethylene, using H2SO4 as catalyst. However, the latter proved ineffective. Ipatieff achieved a success by using ZnCl2 and AlCL as catalysts (Ipatieff, 1945; Butlerov, 1953). In his later works, he used multifunctional catalysts in cracking, reforming, and other cases of oil refining; developed numerous industrially important processes such as the synthesis of polymer benzenes from gaseous olefins (a residue of oil cracking), etc.
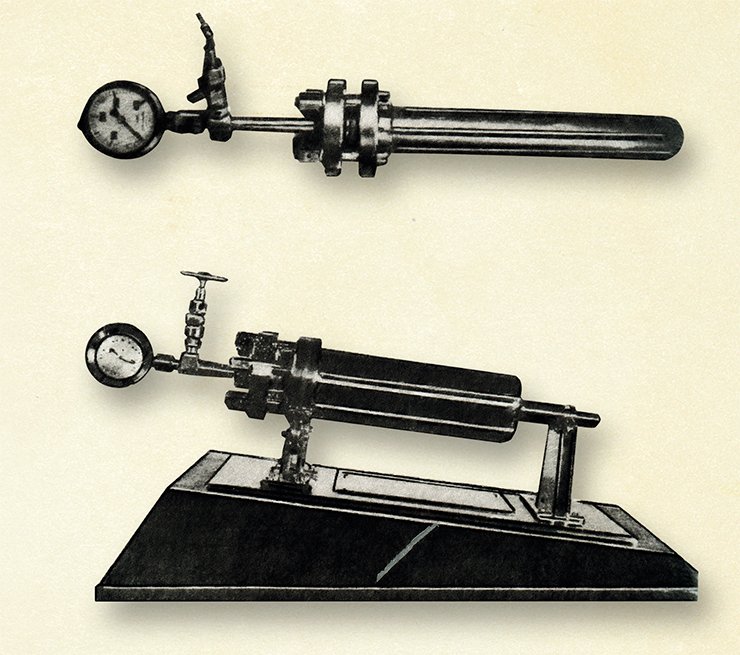 I experimented for a whole year before I constructed an apparatus suitable for high-pressure work. The closure of the bomb or autoclave was achieved by means of a special gasket of heat-treated red copper between two knife edges, one on the top of the bomb and the other on the bottom of the cover. The knife edges cut deep into the soft gasket when the cover was bolted down. Tens of thousands of experiments in Russia and abroad proved that this method of closure was the best for laboratory purposes. It is safe up to 500° C and 450 atmospheres. At lower temperatures, with special steels, this apparatus held a pressure of 1,300 atmospheres, in one case for a whole month. Instead of steels other alloys could be used for the bomb, such as stainless steel, or phosphorous bronze. The gasket can also be made of different metals. When it is necessary to avoid contact of the reactants with the body of the bomb it is possible to use liners of glass, copper, or silver, equipped with capillary tips. For example, I used silver liners and silver gaskets when I studied the pressure oxidation of phosphorus by water to obtain phosphoric acid. Adapted from: (Ipatieff V.N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
I experimented for a whole year before I constructed an apparatus suitable for high-pressure work. The closure of the bomb or autoclave was achieved by means of a special gasket of heat-treated red copper between two knife edges, one on the top of the bomb and the other on the bottom of the cover. The knife edges cut deep into the soft gasket when the cover was bolted down. Tens of thousands of experiments in Russia and abroad proved that this method of closure was the best for laboratory purposes. It is safe up to 500° C and 450 atmospheres. At lower temperatures, with special steels, this apparatus held a pressure of 1,300 atmospheres, in one case for a whole month. Instead of steels other alloys could be used for the bomb, such as stainless steel, or phosphorous bronze. The gasket can also be made of different metals. When it is necessary to avoid contact of the reactants with the body of the bomb it is possible to use liners of glass, copper, or silver, equipped with capillary tips. For example, I used silver liners and silver gaskets when I studied the pressure oxidation of phosphorus by water to obtain phosphoric acid. Adapted from: (Ipatieff V.N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
In 1908, Ipatieff successfully presented at the University of St. Petersburg his academic dissertation “Catalytic Reactions under High Temperatures and Pressures” and received, upon acceptance, the degree of doctor of chemistry. From 1900 to 1917, he published three monographs, two textbooks, and more than a hundred articles in Russian and foreign journals. Alongside his research work, he continued to teach and work at the Artillery Academy; in 1911, he was promoted to the rank of major general; in 1916, lieutenant general. In 1914, he was elected as a corresponding member of the Academy of Sciences (AS); in 1916, a full member (academician) of the Academy of Sciences (AS).

When my father-in-law died suddenly in 1896 he had left a very valuable apartment building in the center of Moscow (Brusovsky Lane) and a large piece of land to my wife and her brother, jointly. This was a great surprise to her and to everyone else; for it had always been assumed that her father would leave the bulk of his estate to his son. But her brother never gave the slightest sign that he had been disappointed. He was a kindly man, very fond of our children; and we were on the friendliest terms. Indeed, he may have been glad that I could help him manage the property. His retiring disposition and lack of business ability made it almost impossible for him to do such things, and it is very likely that if he had been the sole owner, he would simply have sold the property at considerable loss.
During this summer vacation he and I began the construction of a four-story apartment house on the vacant land. A property of this sort produced far more income than government bonds, and it would have been unforgivable to have left the land unused. Building some twenty apartments, we spent about 300,000 rubles ($ 150,000). My uncle Gliky, my mother’s brother, who lived in Moscow, agreed to manage the building and to supervise most of the construction.
In the fall I returned to my investigations and began to prepare butadiene in large quantities. At that time the only method of obtaining it described in the literature consisted in passing the vapors of isoamyl alcohol through a heated tube. The temperature was not specified, but it was apparently around 600 °C. The gases containing a small percentage of butadiene were bubbled through bromine, giving solid butadiene tetrabromide and other bromides. Then the action of zinc and alcohol upon the solid bromide produced gaseous butadiene. Professor Thiele had used this method in preparing butadiene for his experiments on conjugated olefins, employing an iron tube for the pyrolysis instead of a quartz tube, since the former was more suitable for work at a high temperature. I found that in using this method the yield of butadiene was surprisingly small. I tried to discover the reason for this low yield, and I wondered if it might not be because of the polymerization of butadiene under the high temperature used.
Up to this time no attention had been paid to the nature of the liquid products obtained in this reaction; probably most investigators assumed that they were water and undecomposed alcohol. I separated the other liquid products from the water, dried and distilled them, and discovered that the major constituent was isovaleric aldehyde, the remainder consisting of undecomposed isoamyl alcohol. This interesting observation made me surmise that the aldehyde was formed from the alcohol and that the gas should be largely hydrogen. The gas analysis completely substantiated this supposition and I decided to investigate this newly discovered reaction more thoroughly and to determine the conditions under which it took place. First I tested to see if the decomposition took place in glass or quartz tubes, and for the first time I introduced a Le Chatelier pyrometer into the organic combustion furnace. I found that only the iron tubes decomposed alcohol at 500 °C. into aldehyde, and that alcohol passed through glass and quartz tubes unchanged, unless the temperature was raised to more than 700 °C. In glass and quartz tubes, also, the yield of aldehyde was smaller, and the gases contained, besides hydrogen, carbon monoxide, methane, and ethylene.
I immediately carried out similar experiments with ethyl alcohol as well as with secondary and tertiary alcohols, and found the following: all primary alcohols give aldehydes and hydrogen upon passage through an iron tube; secondary alcohols decompose into ketones and hydrogen; while tertiary alcohols yield neither aldehyde nor ketone but at higher temperatures decompose into the hydrocarbons and water.
Thus it became apparent that iron was a compound which caused the decomposition of alcohol without undergoing any change itself; in other words, it was a catalyst.
Adapted from: (Ipatieff V. N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
The works carried out by Ipatieff before 1917 had established the groundwork for thermochemical and thermocatalytic conversion of hydrocarbons as well as petrochemical synthesis in a wide range of temperatures and pressures, leading to the discovery of new research areas in inorganic chemistry. Subsequently, in the United States, he built on and enriched these studies, putting them to practical use by introducing catalytic cracking for the industrial production of high-octane motor fuel and other important products, including those used to manufacture most of the modern polymer materials (Ipatieff, 1959; Loktev, 1991).

Adapted from: (Ipatieff V.N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
Ipatieff was the first to use mixed (i. e., promoted) catalysts of one type (e. g., hydrogenating) and of different types (e. g., hydrogenating and dehydrating), which dramatically increased the potential for heterogeneous catalysis and allowed directional changes in the properties of catalysts. Ipatieff’s research in organic catalysis revealed so many unknown aspects of catalytic reactions that it necessitated a revision of the existing and the development of new theoretical views on catalysis (Ipatieff, 1936; Boreskov, 1986).
Rivalry between Ipatieff and Sabatier
Independently of Ipatieff, the same problem attracted the attention of the French chemist Paul Sabatier (1854—1941), who had been studying organic catalysis since 1897. He applied a much simpler experimental procedure, i. e., passed vapors of the original organic substance through a tube, heated to 100—200 °C and filled with finely crushed metal catalyst (Sabatier, 1932). He conducted all his experiments at atmospheric pressure and mainly with metallic nickel powder. Unlike Sabatier, Ipatieff introduced into the laboratory and, subsequently, industrial practice a new factor – elevated pressure – as well as numerous previously unused catalysts, e. g., Fe, Al, Zn, Cr, Ni, Th, etc., in the form of metals, oxides, halides, etc. (Ipatieff, 1936). Of utmost importance was the effect of combined action of catalysts (promotion), discovered by Ipatieff in 1909. His contemporaries almost immediately recognized the significance of his discovery, as clearly stated, e. g., by Eric K. Rideal and Hugh S. Taylor, founders of the catalytic schools in England and the United States (Rideal and Taylor, 1925).

Two issues were put on the agenda of the last meeting: (1) the deprivation of A. E. Chichibabin of the title of full member in the USSR Academy of Sciences and (2) the deprivation of V. N. Ipatieff of the same title.
“Academician A. E. Fersman,” stated the minutes of the meeting, “presented the correspondence that the Academy had maintained for a long time with Academicians V. N. Ipatieff and A. E. Chichibabin regarding their return to work at the Academy of Sciences. V. N. Ipatieff and A. E. Chichibabin systematically evaded giving a definitive answer on this issue. Only recently, in response to a letter from N. P. Gorbunov, Permanent Secretary of the USSR Academy of Sciences, V. N. Ipatieff and A. E. Chichibabin responded that they did not intend to return to work at the Academy of Sciences. V. N. Ipatieff motivated his refusal by stating that he had signed a contract with a private commercial foreign firm, whose directors categorically object to his trip to the USSR…

“The responses from V. N. Ipatieff and A. E. Chichibabin aroused resentment in many organizations and a huge number of scientific workers. After the announcement of Academician A. E. Fersman, the floor was given to Prof. V. V. Ipatieff, who in his own name and on behalf of his sister expressed outrage at the act of his father, V. N. Ipatieff, and said that he considered the actions of V. N. Ipatieff and A. E. Chichibabin to be completely unworthy of the title of a full member of the USSR Academy of Sciences and irreconcilable with the dignity of the Soviet citizen.”
Academician A. E. Fersman announced the draft resolutions on the expulsion of V. N. Ipatiev and A. E. Chichibabin from among the full members of the USSR Academy of Sciences. The President of the USSR Academy of Sciences V. P. Komarov put the drafts to the vote. The following resolution was adopted (for Ipatieff only) by 63 votes in favor, with six abstentions:
“Since 1927, the full member of the USSR Academy of Sciences V. N. Ipatieff has stayed abroad. V. N. Ipatieff informed the Presidium of the USSR Academy of Sciences that he considered it impossible at present to return to his homeland and resume work at the USSR Academy of Sciences because he had signed a contract with a foreign commercial firm. By refusing to return to work at the Academy of Sciences and decisively preferring to work for a foreign commercial firm, V. N. Ipatieff flagrantly violates the basic duty of every citizen of the Soviet Union – to work for the good of their homeland.
“Considering V. N. Ipatieff’s behavior clearly irreconcilable with the dignity of the Soviet citizen, and even more so with the title of an full member of the USSR Academy of Sciences, the General Meeting of the USSR Academy of Sciences, in accordance with § 24 of the Academy Code, decrees that V. N. Ipatieff shall be deprived of the title of full member of the USSR Academy of Sciences.”
Thus, two chemists who lived at opposite ends of Europe independently developed two chemical theories of catalysis: Sabatier’s theory and Ipatieff’s theory (Sabatier, 1932; Ipatieff, 1936). According to Sabatier, the mechanism of catalysis consists in the formation and decomposition of unstable intermediate compounds (intermediates) with a catalyst. Accordingly, the catalyst is a substance capable of entering into such a chemical reaction with the reactants. It is here, in this understanding, that one should search for the root cause of catalysis and use it as a framework for selecting catalysts. Ipatieff, who recognized the crucial role of the unstable intermediates, emphasized the chemical nature of catalysis and the more complex physicochemical picture of heterogeneous catalysis. He wrote, “…since the very beginning of my studies of catalytic reactions, since 1901, I tried, contrary to the views of the outstanding physicochemists, to look for chemism in catalytic phenomena and to look for the cause of catalytic reactions in the chemical function of the catalyst… to explain the catalytic properties of a substance through its inherent chemical properties” (Ibid.). In this respect, Ipatieff considered pressure and temperature as strong factors, which can substantially influence the properties and interaction of all the components in a catalytic reaction. A similar view was shared and developed by Academician Georgy K. Boreskov (1986), an “extramural” student of Ipatieff and the author of the following semiphenomenological definition: “Catalysis is an initiation or acceleration of chemical reactions over certain substances (catalysts) which repeatedly enter into an intermediate chemical interaction with reagents and restore their chemical composition after each cycle of this interaction.” Without delving into the current state of the theory of catalysis, which has not been finalized yet, I now return to the rivalry between Ipatieff and Sabatier.
From 1913 on I became more and more interested in the actual application of my scientific knowledge to the needs of industry. Unfortunately, the Russian chemical industry was too immature to use the scientific discoveries even then available. I still did not bother to take out patents, and once told one of my friends that I was a scientist and wanted complete freedom in my work, which I would not have if I had to be concerned with patents. Had I been a German chemist I should probably have been infected by the same patent disease as were others.The German chemical industry made full use of my data at no cost to itself. The first man to utilize my investigations on hydrogenation and destructive hydrogenation under pressure was the German engineer, Bergius, who in 1913 took out the first patents on the conversion of tars and certain types of solid fuel into low-boiling hydrocarbons which could be used as gasoline. His reliance upon my work was obvious; and Kling, the French engineer, correctly pointed out the priority of my work in this field, though Bergius certainly deserves the credit for applying my method in the decomposition of the organic compounds found in tars and coal.
Dr. Willstätter in his review of my book Catalytic Reactions at High Temperatures and Pressures (New York, 1936), while speaking of the problem of applying catalysts and pressure in the chemical industry – the synthesis of ammonia by Haber and that of methyl alcohol by Patart and I. G. Farbenindustrie – says: «These new principles were applied very early in Ipatieff’s investigations.»
In the summer of 1913 I appreciated the honor of a visit from Dr. Hibbert, an employee of the American Du Pont Chemical Company. He came to St. Petersburg especially to see my laboratory and to talk to me about my high-pressure investigations. We met again in 1928 at The Hague at the convention of the International Bureau of Pure and Applied Chemistry, and I have since seen him often in the United States. Adapted from: (Ipatieff V. N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946)
In 1912, Sabatier was awarded the Nobel Prize in Chemistry “for his method of hydrogenating organic compounds in the presence of finely disintegrated metals.” His main supporting example was the reduction of ethylene to ethane in the presence of Ag or Ni powders (as catalysts) in H2 medium at 300 °C and atmospheric pressure. The intermediates were, according to Sabatier, the metal hydrides, which gave their hydrogen to ethylene.
The Nobel Committee and the mistake of Russian inventors
In 1915, Ipatieff was nominated for election as a member of the Imperial Academy. The nominators – Academicians P. I. Valden, B. B. Golitsyn, and N. S. Kurnakov – emphatically compared his accomplishments to those of Sabatier and stated in conclusion that the studies carried out by the Russian scientist were “more diverse than the works of Sabatier, who was awarded the Nobel Prize in 1912” (Ipatieff, 1945). One of the nominators, Prof. Valden (Riga Polytechnic), an eminent expert in solution theory, wrote, “If Sabatier received the Nobel Prize for one catalytic reaction only… then Ipatieff’s works undoubtedly deserve the same award because he put catalysts to a much broader use in various reactions… and introduced a completely new high pressure technique, allowing the hydrogenation of substances deemed impossible to work with under Sabatier’s method” (Ibid.; Blokh, 2005).

Another chance at the award he deserved arose two decades later, but Ipatieff again missed the opportunity. The 1931 Nobel Prize in Chemistry went to the German scientists Carl Bosch and Friedrich Bergius “in recognition of their contributions to the invention and development of chemical high pressure methods.” Bosch had been nominated seven times since 1915; the total number of nominations he received, including by Albert Einstein, reached 20. Bergius was nominated twice, with one nominator per cycle. However, not a single nomination mentioned the actual pioneer from Russia (Blokh, 2005).
In this respect, I cannot but mention an overlook on Ipatieff’s own part. In his memoirs, he wrote about the International Congress on Industrial Chemistry, held in Strasbourg in July 1928 (more than three years before the Nobel Prize of Bosch and Bergius). At the congress, he made a presentation, elaborating on his earlier studies on hydrogenation under pressure, and said in conclusion, “Bergius’ patents (1911) are entirely based on my works made back in 1903—1904, and my method, which I developed for various organic compounds, was applied in full for hydrogenation of resins and coals” (Ipatieff, 1945; Blokh, 2005). The Society of Chemical Industry, which organized the Congress, duly appreciated Ipatieff’s outstanding scientific contribution. In a solemn atmosphere, a representative of the French government, in the rank of minister, presented to the Soviet delegate the society’s highest award – the Pierre Berthault Medal.
 Why did the Royal Academy of Sciences not commemorate the undeniable contribution of Ipatieff? Of course, it might have been due to political preferences, i. e., a disapproval of Ipatieff active political engagement in the first years of Soviet power, when he acted as the leader of chemical science and industry in the young Soviet Republic; when he met personally on numerous occasions with Vladimir Lenin; when he was, in fact, a member of the government, etc. However, the decisive factor appears to have been a pure formality, i. e., the lack of a duly executed patent (Blokh, 2005). Here, the scientist repeated the common mistake of Russian inventors who forgot to timely patent their discoveries. If he had obtained a patent, the Swedish experts would have hardly bypassed him and the members of the Nobel Committee, even in the absence of a nomination, could have used their right to nominate their own candidate on the last day of submission of nominations for the current year. I can only add that the Nobel Prize winner Bergius himself admitted subsequently that his method of destructive hydrogenation of coal to liquid motor fuel was entirely based on Ipatieff’s work (Ibid.).
Why did the Royal Academy of Sciences not commemorate the undeniable contribution of Ipatieff? Of course, it might have been due to political preferences, i. e., a disapproval of Ipatieff active political engagement in the first years of Soviet power, when he acted as the leader of chemical science and industry in the young Soviet Republic; when he met personally on numerous occasions with Vladimir Lenin; when he was, in fact, a member of the government, etc. However, the decisive factor appears to have been a pure formality, i. e., the lack of a duly executed patent (Blokh, 2005). Here, the scientist repeated the common mistake of Russian inventors who forgot to timely patent their discoveries. If he had obtained a patent, the Swedish experts would have hardly bypassed him and the members of the Nobel Committee, even in the absence of a nomination, could have used their right to nominate their own candidate on the last day of submission of nominations for the current year. I can only add that the Nobel Prize winner Bergius himself admitted subsequently that his method of destructive hydrogenation of coal to liquid motor fuel was entirely based on Ipatieff’s work (Ibid.).
Note that Ipatieff’s name appeared again in the Nobel Committee’s records in 1949, when he was nominated by Profs. Georges Dupont and Louis Hackspill (Ibid.). But at that time, Ipatieff was way past his prime. He truly could have become the first Russian winner of the Nobel Prize in Chemistry, but he did not, and the primary reason was the astonishing neglect of Russian scientists towards their fellow compatriots.
Life in the USA: Ipatieff’s technology at thousands of industrial plants
Ipatieff was one of those rare scientists who combine in themselves an excellent theorist with an experimenter or even manager, capable of building tomorrow a plant based on a new technological method that he has just discovered today. His fundamental studies wend hand in hand with their practical application. Thus, in 1913, he supervised the first successful hydrogenation of fats in Russia, performed at the Nevsky Stearin Plant. He advised the firms run by the Nobel brothers (Robert and Ludvig, brothers of Alfred Nobel, who founded the Nobel Foundation), Dupont’s firm in the United States, etc. It is this combination of a theorist with a practicing businessman that explains his successful leadership in chemical science and industry during World War One under Nicholas II of Russia and in the first years of Soviet power under Lenin.

The collection includes materials from state archives: the Archive of the Russian Academy of Sciences (RAS), the St. Petersburg Branch of the RAS Archive, and the Russian State Archive of Economics, which have been made publicly available for the first time. The collection addresses a broad readership with an interest in the history of Russian and world science
I will not dwell on Ipatieff’s dramatic biography, which is detailed in his own memoirs (Ipatieff, 1945, 1959) and, even more fully, in the articles published by his students (Orlov, 1927; Haensel, 1940; Pines, 1967, 1981, 1983, 1992; Razuvaev, 1988) and researchers of his work (Loktev, 1991; Kuznetsov, 1991, 1992; Zal’tsberg, 1992; Fenelonov, 2017). You can find this information in the introduction. I will only recall that in the last years of the reign of Emperor Nicholas II, Ipatieff wore the titles of Academician and lieutenant general, and under Lenin, he supervised the reconstruction and development of chemical industry and was a member of the Presidium of the Supreme Council of National Economy (SCNE) and the State Planning Committee, i. e., a member of the government. However, after Lenin’s death, he was gradually ousted from his government posts; in 1926, his membership in the SCNE Presidium was revoked without explanation; he was deprived of the right to participate in its meetings even in an advisory capacity and dismissed from the leadership of chemical industry in the line of the Red Army (he learned about that from the newspapers). Ipatieff received increasingly more summons to the OGPU (Joint State Political Directorate) because of denunciations recalling his generalship, his close ties to Nicholas II, his connections with Leonid Trotsky and other saboteurs and “enemies of the people” (Ipatieff, 1945, 1959). He was expecting an inevitable arrest because many of his colleagues, students, and close acquaintances had been arrested (and even shot). In 1930, in view of the circumstances, he was allowed to go with his wife to Germany for treatment and participation in an international congress. The trip was initially planned for a year but then was extended for another three years. He sought treatment in Germany, France, and England, but was able to undergo a successful surgery for throat cancer only in the United States, in Chicago. There he settled in a modest hotel, where he spent the rest of his life. In Chicago, Ipatieff began to teach a lecture course on catalysis and, simultaneously, conduct experiments in a laboratory perfectly equipped for him by the Universal Oil Products Company. Until 1937, he regularly sent to the Soviet Union the results of his works performed in the United States; purchased and sent, at his own expense, scientific equipment for the high-pressure laboratory; paid for foreign trips of the researchers. In 1936, however, the USSR government and USSR Academy of Sciences demanded categorically his immediate return. He refused to come back, citing his age and the contracts he had signed; he argued that he had organized the transfer of his scientific results to the Soviet Union, etc. On December 26, 1936, Ipatieff was expelled from the USSR Academy of Sciences, and on January 5, 1937, he was deprived of the USSR citizenship and forbidden from entering the country (Ipatieff, 1959). His contacts with the Leningrad laboratory also ceased. The last work that Ipatieff made for Russia (and the whole world) was his monograph Catalytic Reactions at High Temperatures and Pressures, which simultaneously appeared in 1936 in the Soviet Union and United States in Russian and English, respectively (Ipatieff, 1936). This monograph summarized his fundamental scientific results, and it became the reference book for catalytic chemists throughout the world. But in the Soviet Union, it was forbidden to even refer to it.

In the United States, Ipatieff successfully continued the research that he had begun in Russia. In 1936, he was the first chemist to propose catalytic cracking, which allowed a much higher yield of gasoline in the processing of oil or coal (Ipatieff, 1959). The detailed investigation of the alkylation and polymerization reactions during cracking led to his most famous invention – high-octane gasoline and other motor fuels. Ipatieff’s detailed studies in the field of polymerization made it possible to control the composition of cracking products, obtain unsaturated hydrocarbons of a given composition, and, as a result, organize the production of a wide range of polymers and plastics, without which modern civilization would be impossible. Ipatieff also proposed to increase the octane number of gasoline from 60—70 to 100 by adding isopropylbenzene (cumene), which forms during the catalytic cracking of certain fractions. Another way is the treatment of cracking products with dry phosphoric acid, a catalyst in the form of H3PO4 on an inert carrier. Ipatieff was also one of the first to suggest the currently popular Pt/Al2O3 catalysts, whereby highly dispersed platinum is deposited on a carrier of porous alumina. In some cases, the catalytic activity is boosted by preliminary treatment of the carrier with HF vapors (Ipatieff, 1959; Kuznetsov, 1992).

Ipatieff works gave rise to many industrial processes that remain in broad use today. These include the production of motor fuels of various grades, including high-octane gasoline (from oil, coal, etc.); synthetic lubricating oils and additives; solid fats from liquid vegetable oils; hydrogen and synthesis gas from natural gas, etc. This list can be multiplied many times. Today, thousands of oil refineries as well as petrochemical and chemical plants worldwide use in their technology high pressures and the catalysts proposed by Ipatieff.
Since 1947, the American Chemical Society has been awarding the Ipatieff Prize every three years for outstanding experimental work in the field of catalysis and chemistry of processes at high pressures. The Russian Academy of Sciences established a similar award in 1994.

Leading a successful life in the United States, Ipatieff always cherished a devotion to his motherland – Russia – to its culture, language and people. In the United States, he never owned a car or an apartment. He believed that way he would have admitted that he had settled. He thought that he lived and worked there temporarily (for 21 years!) and hoped for a return. During the war, he was touched to the depth of his soul by the victories and defeats of the Red Army and the suffering of the Russian people. Together with Sergei Rachmaninoff and other emigrants, he established a fund to support the Red Army and the people of the Soviet Union. Ipatieff dreamed of rendering his support personally, at least as an experienced military chemist of World War One. During the war, he made three attempts to return to the Soviet Union, wrote applications and tearfully begged Andrei Gromyko, the USSR ambassador in the United States, to help him, but he was turned down all three times (he made his last attempt in 1951, but it was hindered by his illness and death on November 29, 1952).
We would understand many aspects of Ipatieff’s complicated biography if we treated his patriotism as civic sense, based on love and devotion to his motherland and his people and on responsibility to the ancestors and descendants, without any special attitude for the existing government and state, which are the basis of state patriotism. State is a more transient notion than those of nation or people. Therefore, Ipatieff remained loyal to the Russian people both when he abandoned his loyalty to Nicholas II and during his emigration to the United States.
Ipatieff’s greatest merit is carved in ultimately concise words on his tombstone: “In Memory of Russian Genius Vladimir Nikolaevich Ipatieff. The Inventor of Octane Gasoline.”
Ipatieff’s best known Russian students are Academician G. A. Razuvaev, RAS Corresponding Member A. D. Petrov, Profs. B. N. Dolgov, A. V. Frost, V. V. Ipatiev, M. S. Nemtsev, etc. His students abroad are Herman Pines, Robert L. Burwell, and Vladimir Haensel (United States); Jean E. Germain (France), etc. The well-known chemists Aristid von Grosse, Louis S. Kassel, Ralph C. Olberg, Carl B. Linn, as well as many others, also consider themselves as part of Ipatieff’s school. Academician Boreskov, founder of the Institute of Catalysis (Novosibirsk) also considered himself his student. And the students of these scientists are, in turn, Ipatieff’s scientific grand- and great-grandsons.

By a twist of fate, Russia presented the United States with many great scientists, such as G. A. Gamov (1904—1968), a theoretical physicist, astrophysicist, and popularizer of science, the author of the CMB concept and the idea of the triplet genetic code; V. K. Zvorykin (1889—1982), the inventor of the electron microscope and modern television; I. I. Sikorsky (1889—1972), one of the “founding fathers” of modern aviation, the author of the first four motor planes and helicopters, amphibious aircraft and flying boats. And a rightful place in this Group of the Great belongs to Vladimir Nikolaevich Ipatieff.
References
Blokh A. M. Sovetskii Soyuz v inter’ere Nobelevskikh premii (Soviet Union in the Interior of Nobel Prizes), Moscow: Fizmatgiz, 2005 [in Russian].
Boreskov G. K. Geterogennyi kataliz (Heterogeneous Catalysis), Moscow: Nauka, 1986 [in Russian].
Butlerov A. M. Sochineniya v 3-kh tomakh (Writings in Three Volumes), Moscow: AN SSSR, 1953, V. 1, P. 71 [in Russian].
Fenelonov V. B. Ipatieff, an outstanding scientist of the 20th century, one of the founders of heterogeneous catalysis // Kataliticheskii Byulleten’. 2017. V. 84. N 4. P. 4—19 [in Russian].
Fenelonov V. B., Mel’gunov M. S. Texturology in Surface and Nanomolecular Catalysis by ed. R. Richards, Taylor&Francis, Boca Raton, USA, 2006, P. 257—336.
Haensel P. V. N. Ipatieff in Russia // The Chemical bulletin (ACS Chicago Section). 1940. V. 12. N. 4. P. 109—113.
Ipatieff V. N. My life in the United States; the Memoirs of a Chemist. Northwestern University Press, 1959.
Ipatieff V. N. Sesquicentennnial Celebration , Northwestern, Chicago, Sept. 7, 2017.
Ipatieff V. N. Zhizn’ odnogo khimika. Vospominaniya (The Life of a Chemist. Memoirs), New York, 1945 [in Russian] (the first Russian reedition: Academician V. N. Ipatieff. In two books. Moscow: Kalvis, 2011); Ipatieff V. N. et al. The Life of a Chemist: Memoirs of Vladimir N. Ipatieff, Stanford: Stanford Univ. Press, 1946
Ipatieff V. N. Kataliticheskie reaktsii pri vysokikh temperaturakh i davleniyakh (Catalytic Reactions at High Temperatures and Pressures), 1900—1933. Moscow, Leningrad: AN SSSR, 1936 [in Russian]; Ipatieff V.N. Catalytic Reactions at High Temperatures and Pressures, Macmillan, 1936
Ipatieff V. N., Sapozhnikov A. V. Kurs neorganicheskoi khimii (A Course in Inorganic Chemistry), 7th ed., Moscow: Mosk. Nauch. Inst., 1920 [in Russian].
Kuznetsov V. I. Vicissitudes of work of Academician Vladimir N. Ipatieff // Repressirovannaya nauka, Leningrad: Nauka, 1991, P. 367—376 [in Russian].
Kuznetsov V. I., Maksimenko A. M. Vladimir Nikolaevich Ipat’ev, 1897—1952, Moscow: Nauka, 1992 [in Russian].
Loktev S. M. Akademik Ipat’ev – khimik novogo veka (Academician Ipatieff: A chemist of the New Century), Moscow: Znanie, 1991 [in Russian].
Morachevskii A. G., Firsova E. G. Life and work of Academician Vladimir N Ipatieff (dedicated to his 150th birthday anniversary) // Nauchno-tekhnicheskie vedomosti SPbPU. Estestvennye i inzhenernye nauki, 2017, V. 23. N 3. P. 165—172 [in Russian].
Orlov N. A. Vladimir Nikolaevich Ipatieff // Priroda. 1927. N 5. P. 330—342 [in Russian].
Razuvaev G. A. Stories without details // Khimiya i zhizn’. 1988. N 2. P. 15—19 [in Russian].
Rideal E. K., Taylor H. S. Catalysis in Theory and Practice, London: Macmillan, 1925.
Sabatier, P. La catalyse en chimie organique, C. Béranger, 1913.
Teleshov S. V. Removing the sands of oblivion (to the 150th birthday anniversary of Academician V. N. Ipatieff) // Kataliticheskii Byulleten’. 2017. V. 84. N 4. P. 20—34 [in Russian].
Zal’tsberg M. Portraits: Three lives of Academician Ipatieff // Khimiya i zhisn’. 1992. N 10. P. 78—85, N 11. P. 25—31, N 12. P. 17—25 [in Russian].
The editorial staff of SCIENCE First Hand thanks Dr. Sci. (History) I. V. Tunkina, Acting Director, St. Petersburg Branch, Archive of the Russian Academy of Sciences (SPbB ARAS, St. Petersburg); Cand. Sci. (History) E. N. Gruzdeva, Head of Department and Reading Room, SPbB ARAS; O. S. Bystrova, Director of the Kalvis Publishing House (Moscow); V. V. Agafeev, Director of Honeywell UOP (Moscow); and Chris Nicholas, Engineer / Scientist Principal R & D, Honeywell UOP (Des Plaines, USA) for their invaluable support in preparing the illustrations for this articles









