The Postgenomic Era. Breeding for Accelerated Senescence
Creation and characterization of the biological models for socially significant diseases is an approach to clarification of their etiology and pathogenesis and elaboration of new treatment and prevention methods
The number of genetic models is growing in an avalanche-like manner, and the most abundant among them are monogenic variants, namely, transgenic animals, animals with a knockout gene, or animals carrying certain mutations. This approach brings us closer to understanding the contribution of a particular gene to the corresponding trait yet fails to reproduce the phenotypic manifestation of polygenic diseases. This is most true for the “elderly” diseases, which are frequently developing in humans in parallel with and on the background of a set of aging manifestations, which appear at a significantly different time moments. Creation of the models simulating such situation is a complex problem; however, this is an optimal approach to searching for and testing new therapeutic and prevention methods.
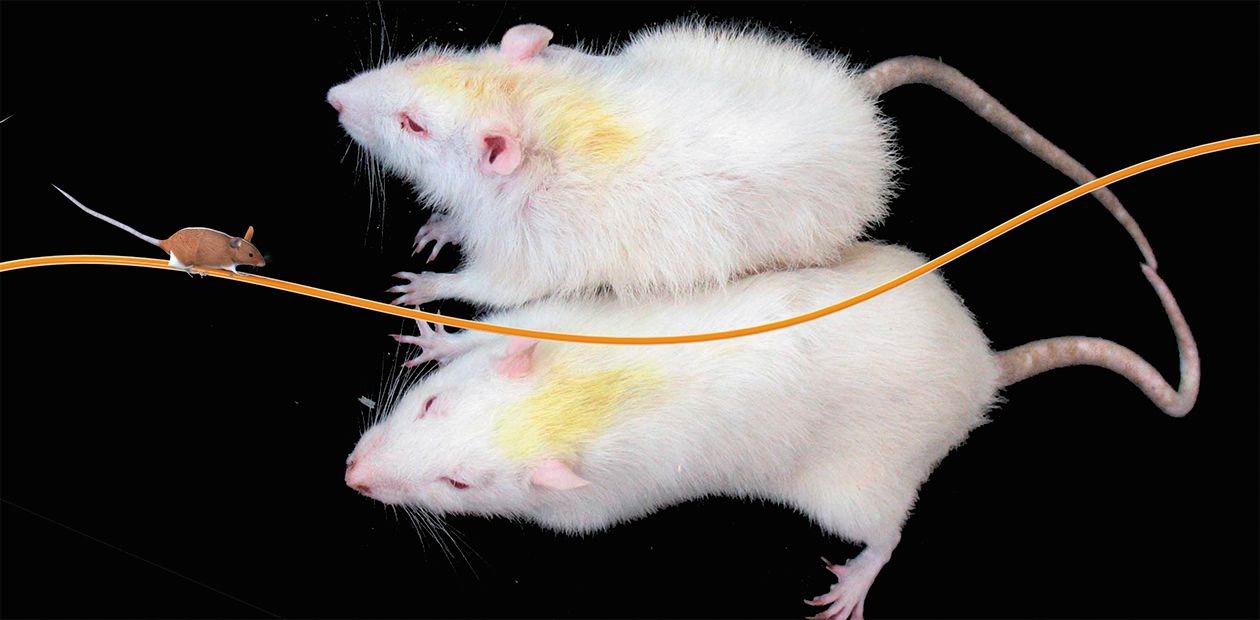
In fact, a single accepted model of accelerated senescence—SAM (senescence accelerated mouse)—exists in the world; it was created by Japanese scientists and is currently represented by 12 substrains differing in the pattern of disorders in emotional sphere, circadian rhythms, the degree and nature of cognitive abnormalities, and other aging manifestations. According to our studies, the rat strain OXYS, created at the Institute of Cytology and Genetics SB RAS in the 1970s by the team of Academician R. I. Salganik, can be a full-fledged model for accelerated senescence and related diseases. The development of a set of acceletared senescence signs in these animals resulted from a directed selection of Wistar rats for early spontaneous cataract.
In the first five generations, this disease was provoked by a galactose-enriched diet. By the moment, we have the 82nd generation of OXYS rats with a spontaneously developing cataract and the accelerated aging syndrome inherited in a linked manner. Accelerated aging manifests itself in a 28% shorter lifespan as compared with the intact Wistar strain and in an early development of phenotypic aging changes and age-dependent diseases on the background of an early involution of the thymus and a decrease in the activity of the T-cell component of the immune system. In tight collaboration with a team from the Novosibirsk Institute of Regional Pathology and Pathomorphology (Siberian Branch of the Russian Academy of Medical Sciences), Helmholtz Institute of Eye Diseases (Moscow), and Novosibirsk Institute of Traumatology and Orthopedics, we succeeded in demonstrating that the changes developing in OXYS rats corresponded to the main criteria for the models of such diseases, namely, senile cataract, age-related maculodystrophy, and osteoporosis. Manifestations of these diseases are reproduced at clinical and morphological levels and respond to a standard therapy. This model is efficiently used for a rapid assessment of new diagnostic, prevention, and therapeutic tools for various age-related diseases and prevention of accelerated senescence.
Collaboration with colleagues from the Moscow State University under the “Skulachev Ions” project has considerably expanded basic research into molecular genetic nature of accelerated senescence. This project was aimed at solving applied problems, namely, the development of pharmacological preparations able to act directly on the cell power stations, mitochondria, interfering with the aging programs. Four months of the trials using OXYS rats were sufficient to demonstrate the ability of the mitochondrion-targeted antioxidant SkQ (“Skulachev ions”), synthesized by the team of Academician V. P. Skulachev, to prevent and (or) hinder accelerated aging and development of the related diseases in OXYS rats. The trials, which took one year, demonstrated a unique therapeutic potential of this preparation and its ability not only to prevent and hinder the development of cataract and retinal dystrophy, but also to alleviate the already developed pathological changes to their complete disappearance.