The Postgenomic Era. Creation of Transgenic Animals
Transgenic organisms are no rarity now and their value for biology and medicine is difficult to overestimate. An artificial switch-on of almost any gene of interest allows physiologists to study various abnormalities in the organism homeostasis, immune system, embryogenesis, and so on in the experiments with transgenic animals
Biomedical research is mainly focused on the creation of a wide range of the models for human diseases, including atherosclerosis, diabetes, hypertension, Alzheimer’s disease, retinoblastoma, and various cancers. Transgenic animal models, as a rule, mouse models, make it possible to study the mechanisms underlying the development and treatment of human diseases.
Transgenic mice are also an indispensable model system for testing genetic constructs before creating transgenic farm animals—bioreactors capable of producing human proteins with milk.
Granulocyte colony-stimulating factor (G-CSF) is a valuable hematopoietic stimulator, widely used in clinical practice in blood transfusion and treatment of the consequences of radio- and chemotherapies in oncology (Hobel et al., 2003). It has been clinically demonstrated that G-CSF therapy prevents and cures infections in immunoincompetent patients having a positive effect on the blood cells. G-CSF is currently used for various purposes in the so-called regenerative medicine. It has been reported that G-CSF improves the heart muscle functions and reduces the mortality after acute myocardial infarction when the patient’s blood stem cells are engrafted at the infarction site. G-CSF is synthesized in monocytes, fibroblasts, and endothelial cellsThe most widespread method used for introducing transgenes is microinjections into the pronuclei (male and female nuclei) of the zygote at the moment when a spermatozoid has just entered the ovum and the pronuclei are preparing to fuse. This method implies injection of exogenous (foreign) DNA solution (containing 500—1000 gene copies) into the male pronucleus with the help of a micromanipulator equipped with a glass capillary. This procedure is monitored with a microscope equipped with special optics providing a 3D imaging of pronuclei.
Three genetically engineered constructs able to provide the synthesis of biologically active granulocyte colony-stimulating factor (G-CSF) in the milk of transgenic mice were produced. The constructs contained the human G-CSF fused with the regulatory sequences of cow or goat alpha-S1 casein gene whose length varied from 721 to 3387 base pairs (bp). This made it possible to switch on the set of regulatory sites, from minimally to maximally necessary, that compose the intricate physiological mechanism providing activation of the mammary grand functions, such as the receptors for hormonal stimulation of lactation and pregnancy. The microinjections of recombinant DNA into mouse zygotes allowed over 10 transgenic animals to be produced; each animal gave rise to an individual mouse strain. Over 400 progenies of these progenitors were assayed for the transgene inheritance and the production and biological properties of the recombinant human G-CSF. It was found that the transgene was inherited in a Mendelian manner as any natural gene and stably expressed in the mammary glands of transgenic lactating females of all strains. The concentration of human G-CSF in milk varied from 0.1 mg/ml to 1000 µg/ml. The transgenic animals carrying the “shortest” regulatory region (721 bp) displayed human G-CSF in the blood serum, indicating that the transgene was ectopically expressed, i. e., the human protein was synthesized not only by mammary gland cells, but also by the cells of other internal organs. This was an undesirable complication, as this transgenic protein is a strong stimulator of hematopoiesis and can impair the health of a transgenic individual. The milk of all transgenic animals induced the development of hematopoietic granulocyte colonies in the culture of human umbilical blood cells, and its activity was comparable with the activity of natural human G-CSF preparations. The third construct with the longest regulatory region (3387 bp) appeared the most promising in overall characteristics and was used to create transgenic goatsThe integration efficiency of transgenes into the genome, i. e., the fraction of transgenic animals in the total number of the progenies born after this procedure, is almost independent of the animal species, amounting to 5—15% in mice, 10—15% in pigs, 10% in rabbits, and 5—10% in sheep, goats, and cows.
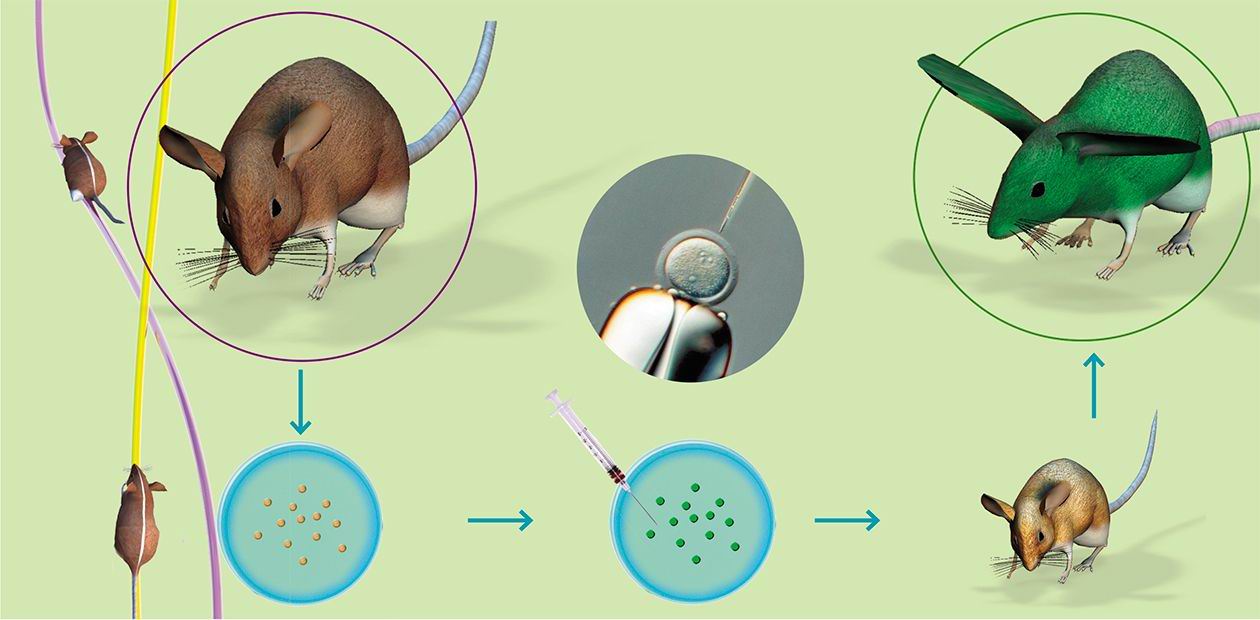
Creation of the transgenic animals comprises the following stages:
(1) Isolation of zygotes from hormonally stimulated females;
(2) Microinjection of the DNA (genetic constructs) solution into the male pronucleus of zygote;
(3) Transplantation of injected zygotes to the recipient females prepared for carriage;
(4) Analysis of the newborns for the presence of transgene by polymerase chain reaction (PCR analysis);
Interferon, blood clotting factor IX, serum albumin, several immunoglobulins, and many other proteins, already recommended for creation of milk bioreactors, form an incomplete list of the pharmacologically valuablehuman proteins(5) Analysis of the transgenic animals for inheritance of transgene, expression of the recombinant protein, and its biological activity, as it is necessary to find out whether the inserted transgene is active and inherited, the protein product displays all biological properties of the natural analog, and the production level fits the biotechnological purposes; and
(6) Creation of the strain (herd) of transgenic animals for research or biotechnological purposes.
Animal bioreactors
Production of therapeutically valuable human proteins is a promising direction in modern biotechnology. The strategy for creation of dairy cattle as bioreactors involves insertion of a combined genetic construct carrying the human DNA sequence encoding a necessary protein into the region controlled by the regulatory elements of a cow, goat, or sheep “milk gene”. According to this strategy, thus created transgenic animals are able to synthesize large amounts of the corresponding human protein exclusively in the mammary gland and secrete it into milk, which in turn becomes the source for isolating this human protein.
The examples of successful application of this technology include creation of transgenic goats, sheep, rabbits, and pigs producing the human proteins antitrypsin, serum C reactive protein, antithrombin, lactoferrin, calcitonin, and others (Wall et al., 1997; Rudolph, 1999; Gol’dman et al., 2002).
The pharmaceutical market of the recombinant proteins produced from the milk of transgenic farm animals is estimated as one billion dollars and is predicted to reach 18.6 billion dollars by the year 2013 (Niemann et al., 2007).
The new genetically engineered preparations are, as a rule, tested using transgenic mice, as the majority of large animals, potential producers of such preparations, have long reproductive period and are expensive to keep. The analysis of constructs in mice provides for a quick selection of the most promising variants with respect to both the production level of target protein and its biological properties.
The projects on creation of transgenic bioreactors usually comprise three stages: production of the genetic construct with a human gene under the control of regulatory regions of a “milk gene” (for example, casein), testing of this construct in transgenic mice, and, eventually, insertion of the selected constructs into the genome of dairy cattle.
Creation of an efficient producer is a stroke of good luck and a guarantee that a herd of its offspring will be produced in relatively short terms to supply the market with valuable pharmaceutical product. A laboratory is organized for such a herd to isolate and purify the recombinant protein; this protein is conveyed to the corresponding pharmaceutical institutions for testing, performance of preclinical and clinical trials, and approval of its use in medical practice.
The experience of leading pharmaceutical companies of the United States, Canada, and United Kingdom demonstrates that the average life of such projects is about 10 years given a high funding level. Unfortunately, Russia practically lacks such technologies as well as an infrastructure for their implementation; this means that the biologically valuable human proteins, the number of which reaches 60 (Rudolph, 1999), will be purchased abroad.
It is a shame, the more so that Russian scientists have created and tested on transgenic mice several efficient gene constructs for biotechnological applications (Zavadskaya et al., 2001; Dvoryanchikov et al., 2005; Gol’dman et al., 1998; Zakharova et al., 2006). However, there are no modern biotechnological farms in Russia, and these developments are hence not used to a full degree.
The two following biotechnological projects currently being implemented in Russia are actually an exception.
The joint Russian—Brazilian project conducted by the Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences; Institute of Molecular Genetics, Russian Academy of Sciences; Federal University of Rio de Janeiro (UFRJ); and State University of Ceara (UECE) succeeded in creating a transgenic goat carrying the human G-CSF (granulocyte colony-stimulating factor) gene in July 2006; the corresponding genetic construct was earlier tested in transgenic mice (Dvoryanchikov et al., 2005). The paper on creation of the transgenic goat was published in the Annals of the Brazilian Academy of Sciences (Freitas et al., 2007), the experiment was successfully repeated in the fall of 2007, and three transgenic kids were born in March 2008; two of them, Camilla and Augustine, are now growing.
The second biotechnological project with goats, known so far only from popular press, is carried out under a Russian—Belarusian program and focuses on a genetic construct containing human lactoferrin. Lactoferrin is a component of woman’s breast milk and a natural antibiotic, protecting the child from manifold diseases. The government customers for the program are the Russian Federal Agency for Science and Innovations and the National Academy of Sciences of Belarus. The main part of this research is performed by the experts from the Institute of Gene Biology, Russian Academy of Sciences, and the Research and Applied Animal Breeding Center with the National Academy of Sciences of Belarus. At the end of 2007, they succeeded in obtaining two transgenic goat kids carrying the human lactoferrin gene.
References
Gol’dman I. L. et al. Lactoferrin: properties and prospects of biotechnological production // Biotekhnologiya. — 1998. — N. 4. —Pp. 3—16.
Gol’dman I. L. et al. Transgenic goats in the world pharmaceutical industry of the XXI century // Genetika. — 2002.—V. 38, N. 1.—Pp. 5—21.
Zavadskaya E. S. et al. Production of recombinant endostatin in the milk of transgenic mice // Genetika.—2001.—V. 37. — Pp. 1207—1212.
Dvoryanchikov G. A. et al. Secretion of biologically active human granulocyte colony-stimulating factor (G-CSF) in milk of transgenic mice // Russ. J. Genet.—2005.—V. 41, N. 10. — Pp. 1088—1094.
Freitas V. J. et al. Production of transgenic goat (Capra hircus) with human granulocyte colony-stimulating factor (hG-CSF) gene in Brazil // Ann. Braz. Acаd. Sci.—2007.—V. 7, N. 4. — Pp. 585—592.
Hubel K. et al. Clinical applications of granulocyte colony-stimulating factor: an update and summary // Ann. Hematol. — 2003.—V. 82.—Pp. 207—213.
Niemann H. et al. Transgenic farm animals: an update // Reprod. Fertil. Dev.—2007.—N. 19.—Pp 762—770.
Wall R. J. et al. Transgenic dairy cattle: genetic engineering on a large scale // J. Dairy Sci.—1997.—V. 80.—Pp. 2213—2224.
Wall R. J. Transgenic livestock: progress and prospects for the future // Theriogenology—1996.—V. 45.—Pp. 57—68.
Zakharova E. S. et al. Transcription and mRNA splicing of the human lactoferrin gene controlled by the regulatory region of the bovine alphaS1 casein gene in the mammary gland of transgenic mice and in mouse embryonic stem cells // Dokl. Biochem. Biophys.—2006.—N. 411.—Pp. 336—338.
Photos by the courtesy of L. E. Andreeva, a participant of the project, candidate of biology, and senior researcher with the Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia