The Tuning Fork for Aging
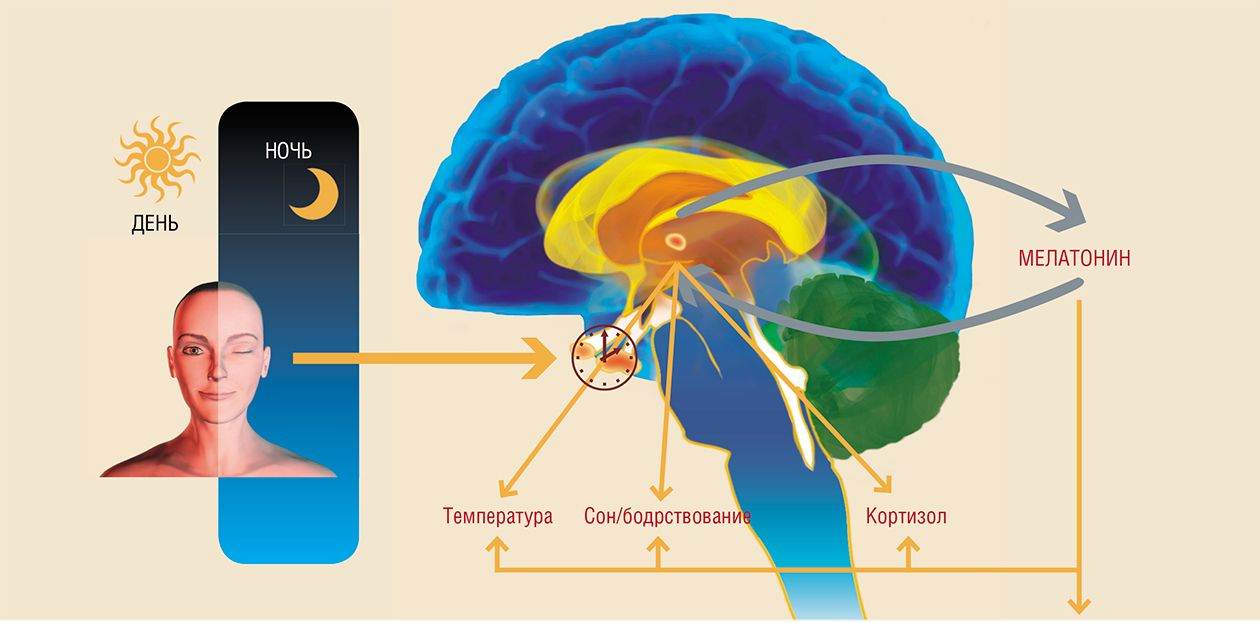
The alternation of day and night, light and darkness is among the most essential natural phenomena on Earth. The rotation of our planet around its axis and concurrently around the Sun ticks away days, seasons, and years of human life. The epiphysis (pineal gland) is the biological clock of the organism, and its hormone, melatonin, may be compared to the balance wheel that regulates the beat of this clock. It is difficult to overestimate the role played by our natural chronograph in the regulation of the body’s physiological rhythms and their adaptation to the environmental conditions...
The honor of this discovery belongs to Aaron Lerner, a Yale dermatologist. In 1953, he succeeded in isolation of the extract from bovine pineal glands that made the color of the frog skin lighter. Lerner and his colleagues processed 250,000 pineal glands to obtain the key component, an active substance identified as N-acetyl-5-methoxytryptamine.
The discoverer gave this new hormone the more romantic name of melatonin (melas is Greek for “black” and tosos is Greek for “labor”) and brought his discovery to the public eye in a one-page paper published in 1958 in the Journal of American Chemical Society.
Our “Sundial”
The pineal gland — a neuroendocrine organ whose main function is transmission of information about environmental light conditions into the body’s internal environment — is found in all vertebrates.
Note that the body contains also the extrapineal melatonin, i.e., melatonin synthesized outside the pineal gland. It was discovered by Russian scientists, N. T. Raikhlin and I. M. Kvetnoi, who found in 1974 that it was generated by the cells of the vermiform appendix. It has been found out that melatonin is also formed in other parts of the gastrointestinal tract as well as in the liver, kidneys, gallbladder, ovaries, placentas, thymus, blood cells (leukocytes and platelets), and so on. The biological effect of extrapineal melatonin is realized directly at the site of its synthesis.
Perhaps, it would be most appropriate to compare the pineal gland to a sundial, where melatonin plays the role of gnomon, the rod used for determining the solar altitude. During the day the sun is high, and the shadow cast is short (correspondingly, the level of melatonin is minimal), whereas at nightfall the synthesis of melatonin by the pineal gland and its secretion into the blood increase. Thus, melatonin concentration follows a circadian rhythm, which is determined by a chronological metronome, the rotation of Earth around its axis.
Within the frame of the body’s circadian rhythm, melatonin sustains the sleep-wake cycle and circadian changes in the locomotor activity and body temperature. Melatonin concentration in the blood reaches its maximum an hour or two before arousal: this is the time when the human sleep is deepest and body temperature is minimum.
Electricity, which came to stay over a hundred years ago, drastically changed the light conditions. The action of light on humans at night, frequently called light pollution, has become an essential part of the modern lifestyle, characteristic of which are numerous serious disturbances of behavior and health, including cardiovascular diseases and cancer. According to the hypothesis of circadian destruction, a long-term use of additional illumination interferes with the circadian rhythm and in-hibits the night secretion of melatonin, decreasing its concentration in the blood.
Melatonin as a Night Security Guard
It has been demonstrated that monochrome blue light of 1.3 lux (or 100 lux of white light) can significantly inhibit the pineal production of melatonin. Volunteers exposed to intermittent illumination in the nighttime over two weeks displayed a considerable decrease in the level of melatonin.
The interplay of positive and negative feedbacks between the function of several, at least nine, main “clock genes”, which underlie the circadian rhythm, determines the molecular clock mechanism of the suprachiasmatic nucleus of the hypothalamus (its neurons transmit the light information to the pineal gland). It has been found that light acts directly on expression of some of the genes in question. In their turn, these genes regulate the functions of the key cell cycle and apoptosis genes. Mutations in certain clock genes have a dramatic effect on many functions of the body, leading to development of various pathologies.
An artificial extension of the photoperiod by just 2—4 hours prolongs the estrous cycle of rodents and may even cause its abnormalities in some cases. Round-the-clock illumination of mice and rats induces a rapid development of the persistent estrus syndrome. Under natural conditions, this syndrome develops at a later age to pass into anestrus, a physiological equivalent of female climacteric.
Ovaries of the rats with persistent estrus display follicular cysts and ovarian interstitial cell hyperplasia and a lack of yellow bodies. Cyclic hormone production, characteristic of a normal reproductive period, is impaired, thereby leading to hyperplasia in the mammary glands and uterus.
All in all, rats in this respect have appeared rather similar to humans. It is known that additional illumination shortens the menstrual period in women with a long (over 33 days) cycle. For example, 60 % of the examined paramedics with a regular menstrual period and frequent night shifts displayed the cycle shorter than 25 days and about 70 % of the test women complained of rare or frequent absence of menses.
Constant exposure to illumination elevates the threshold of the hypothalamus sensitivity to estrogen inhibition,the key mechanism in the aging of reproductive systems in both rat females and women. Thus, additional night illumination enhances an accelerated age-related switching-off of the female reproductive function.
Main functions of the pineal gland in the body:
• Regulation of circadian and seasonal rhythms
• Regulation of reproductive function
• Antioxidant defense
• Antitumor defense
• “Sundial of aging”
Decreases in glucose tolerance and insulin sensitivity were also found in the rats with persistent estrus. In addition, constant light exposure intensifies the lipid peroxidation in animal tissues versus its decrease by melatonin, especially in the brain.
The antioxidant action of melatonin was discovered in 1993 by Russel Reiter, an American scientist. This effect results from a pronounced ability of melatonin to neutralize free radicals, including those formed during lipid peroxidation, as well as from activation of glutathione peroxidase, a powerful endogenous factor in the enzymatic defense against radical oxidation, in the presence of melatonin.
During the last 20 years, it has been found that melatonin plays a most important role in the regulation of immune defense, including antitumor immunity. It has been proved so far that melatonin receptors are present on the membranes of many immunocompetent cells. An ablation of the pineal gland or administration of the drugs inhibiting melatonin synthesis is accompanied by suppressed antibody production, whereas melatonin application stimulates the lymphocyte production of interleukins and y-interferon.
Night Work and Cancer
People working at night or in shifts constitute one-fifth of the total amount of labor in the Unites States and in most EC countries. An evident decline in the health of these workers is tightly connected with sleep disturbances, gastrointestinal and cardiovascular diseases, metabolic abnormalities, as well as potentially increased risk of diabetes.
It has been demonstrated that obesity, high levels of triglycerides and cholesterol, and a low concentration of “antisclerotic” high density lipoproteins (HDL) are observed in this group of workers more frequently than with those working in day shifts. In addition, it has been proved that, along with hypertension and decreased blood fibrinolytic activity and glucose tolerance, these characteristics are the risk factors not only of cardiovascular diseases, but also of malignant tumors. This explains higher mortality rate from malignant tumors among shift workers as compared with those working only in the daytime.
Paramedics who have worked in shifts for over 30 years show a higher risk of breast cancer, which is accompanied by a decrease in melatonin level and elevation in the estrogen concentration in their blood. A high cancer risk is also characteristic of women in air crews and women working in night shifts.
As early as in 1964, German researcher W. Jochle discovered that the number of breast tumors and the resulting mortality of the mice exposed to a round-the-clock illumination was considerably higher than with the animals kept under a conventional photoperiod. An analogous pattern is also true for other tumors.
Two years later, I. O. Smirnova from the Russian Cancer Research Center (Moscow, Russia) found that after a seven-month exposure to constant illumination mammary glands of the overwhelming majority of female rats developed hyperplastic processes and mastopathy. According to A. I. Vinogradova (Petrozavodsk State University, Russia), slightly over half of the female rats exposed to constant illumination reach the age of 18 months whilst in the conditions of a standard photoperiod almost 90 % of the animals manage to live to this age. Moreover, spontaneous tumors are recorded in 30 % of the rats from the experimental group versus 16 % for the group kept under standard illumination conditions.
Similar consequences of constant illumination were recorded in the experiments conducted in our Lab by D. A. Baturin with the female HER-2/neu transgenic mice. These animals displayed a larger number of multiple mammary adenocarcinomas as compared with the female group kept under standard illumination. Note that the carcinogenic effect of constant illumination was proportional to its intensity.
I. K. Khaetsky (Institute of Cancer Problems, Kiev) was the first to report in 1965 a stimulatory effect of constant illumination on the chemically induced mammary tumors in rats. Later, several Russian and foreign researchers demonstrated that constant illumination stimulated various carcinogen-induced tumors in animals.
Moreover, round-the-clock illumination is harmful even if it is not constant. For example, in our joint experiments with D. Sh. Beniashvili, the rats exposed transplacentary to the carcinogen N-nitrosoethylurea were kept in the room with switched-on light over the entire period of their pregnancy and nursing; then the offspring was kept under a standard photoperiod. It was demonstrated that even a short-term exposure of rodents to constant illumination promoted the development of nervous and kidney tumors in response to carcinogen application.
An Antidote for Time?
Thus, epidemiological and experimental research has allowed scientists to find that the ecological and genetic factors disturbing the systemic and/or local circadian rhythm can jeopardize the temporal regulation of cell division and stimulate tumor growth. However, their negative impact on the body extends beyond this.
Note that the level of melatonin production in the body is in no way constant during the lifetime even under favorable ambient conditions. In particular, the majority of physiological parameters in humans 60—74 years old display a positive phase shift of the circadian rhythm of about 1.5—2 hours ahead of time. Secretion of many hormones, body temperature, sleep, and some behavioral rhymes are frequently desynchronized in people older than 75 years. What could be the reason? With aging the pineal gland function is suppressed, along with other functions; first and foremost, this appears in an impaired rhythm of melatonin production and decrease in its secretion level.
Effects of constant illumination:
• Inhibition of melatonin synthesis and secretion
• Increase in prolactin synthesis and secretion
• Elevation in the threshold of hypothalamus sensitivity to estrogen inhibition
• Induction of anovulation and ovarian cysts
• Stimulation of proliferative processes and tumorigenesis in the mammary gland and in the endometrium
• Increase in generation of reactive oxygen species
• Stimulation of atherosclerosis and development of metabolic syndrome
Once the pineal gland is the body’s sundial, it is evident that any changes in the daylight duration must essentially influence its functions and, eventually, the rate of aging. Indeed, several studies have demonstrated that impaired photoperiodicity can lead to a considerable shortening of life.
M. Hurd and M. Ralph, American scientists, have found that the lifespan of the golden hamsters carrying a specific mutation in the gene that is a pacemaker for the suprachiasmatic nucleus of the hypothalamus (these particular signals determine the rhythm of melatonin production) was by 20 % shorter as compared with the lifespan of the control animal group. This effect appeared completely removable by implanting embryonic hypothalamic cells from healthy hamsters into the brain of old mutant animals.
An experimental destruction of the suprachiasmatic nucleus also shortens animals’ life. This is no wonder as in these nuclei acts the set of clock genes mentioned above. An impaired function of one of these genes (Per2) causes premature aging of mice and increases their sensitivity to tumor development. Mutations in another circadian gene (Clock) result in development of obesity and metabolic syndrome as well as in premature disturbances of the mouse reproductive cycle.
Numerous studies have demonstrated that melatonin administration is able to delay aging and increase lifespan of various laboratory animals (fruit flies, flatworms, mice, and rats). In addition, several papers report the ability of melatonin to increase the resistance to oxidative stress and to lessen the manifestations of various diseases connected with age, such as retinal macular dystrophy, Parkinson’s and Alzheimer’s diseases, hypertension, and diabetes mellitus.
All these facts give certain optimism regarding the possible use of this hormone in medical practice. Undoubtedly, further comprehensive clinical trials of melatonin are necessary. As we see it, these trials would essentially expand the range of its administration in treatment and prevention of age-related diseases and, eventually, premature aging.
References
1. Anisimov V. N. Physiological functions of the pineal gland (gerontological aspect) // Russian Physiol. J. — 1997. — No. 8. — Pp. 1—13.
2. Anisimov V. N. Melatonin and its place in the modern medicine // Russian Med. J. — 2006. — V. 14. — No. 4. — Pp. 269—273.
3. Anisimov V. N., Ailamazyan E. K., Baturin D. A., Zabezhinsky M. A., Alimova I. N., Popovich I. G., Beniashvili D. Sh., Manton C. R., Provinciali M., Francesci C. Light regimen, anovulation, and risk of malignant tumors of the female reproductive system: correlation masochisms and prevention // J. Obstetr. Women’s Dis. — 2003. — V. 52. — No. 2. — Pp. 47—58.
4. Anisimov V. N., Vinogradova I. A. Light regimen, melatonin, and risk of cancer development // Vopr. Onkol. — 2006. — V. 53. — No. 5. — Pp. 491—498.
5. Anisimov V. N., Zabezhinsky M. A., Popovich I. G. Melatonin suppresses the large intestine carcinogenesis induced by 1,2-dimethylhydrazine in rats: the effects and putative mechanisms // Vopr. Onkol. — 2000. — V. 46. — No. 2. — Pp. 136—148.
6. Anisimov V. N., Kvetnoi I. M., Komarov F. I., Malinovskaya N. K., Rapoport S. I. Melatonin in Physiology and Pathology of the Gastrointestinal Tract. — Moscow, 2000.
7. Arushanyan E. B. Chronopharmacology at the Border of Centuries. — Stavropol, 2005.
8. Komarov F. I., Rapoport S. I., Malinovskaya N. K., Anisimov V. N. Melatonin in the Norm and Pathology. — Moscow, 2004.