The Tubes are Nano, the Capacitors are Super!
For several years, experts from the Institute of Inorganic Chemistry SB RAS (Novosibirsk) have been working on the methods for synthesis of arrays of directional carbonic nanotubes and studying their structure and characteristics. As an electrode material, nanotubes show great potential for creating new kinds of supercapacitors and accumulators.
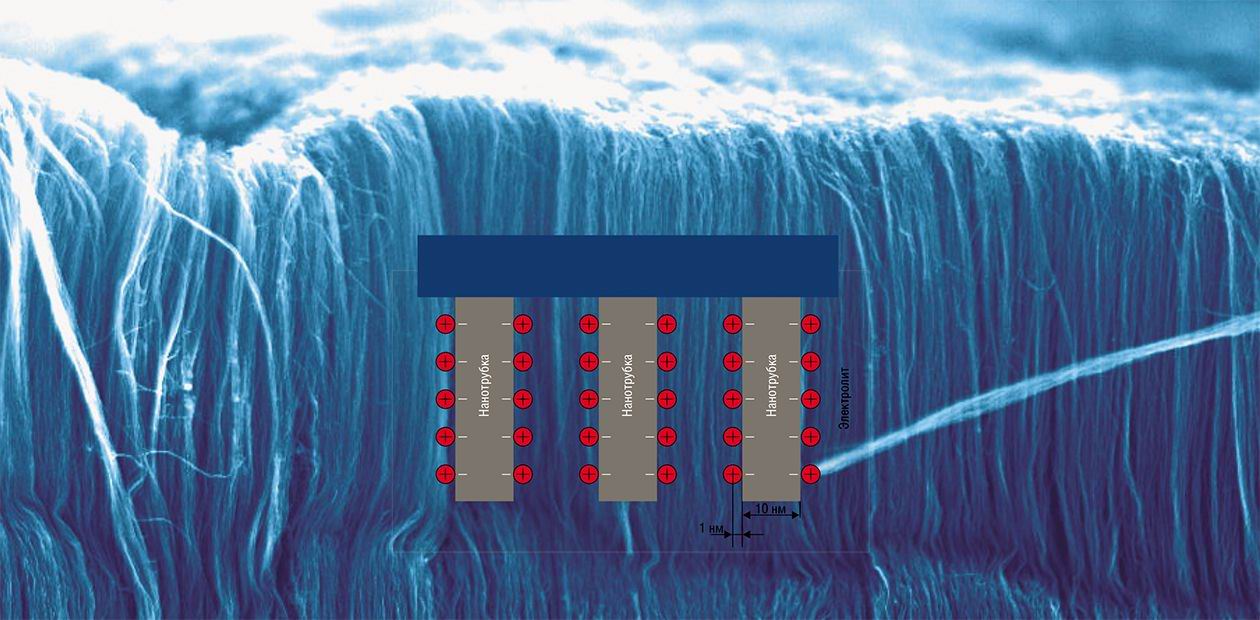
The growing needs of the contemporary technology have resulted in the appearance of a new class of devices—supercapacitors, or ionistors. They have high capacity and accumulate energy in the double electric layer on the surface of a highly porous conductive structure. As opposed to the usual capacitors, the second electrode in supercapacitors is the electrolyte that at 1 V voltage allows a layer of ions to form on the electrode surface. The ions are in the solvate shell composed of water molecules spaced at characteristic intervals of about 1 nm.
It is known that the capacity of an elementary capacitor is in proportion to the area of electrodes and in inverse proportion to the distance between them. Since in an ionistor the distance between the charged surface of the electrodes and the layer of ions of the electrolyte is very short and the specific surface of a porous conductor (for example, activated carbon) reaches 1000—1500 m2/g, the capacity of such a device can exceed 100 Farad/g. To compare, the specific capacity of traditional electrolytic capacitors is one-thousandth of ionisters’ capacity.
Supercapacitors are characterized by high power and low leakage currents, they endure tens of thousands of charge-discharge cycles and can be charged in a short time. They are an effective means for reliable motor starting at low temperatures, as well as in case when the battery is dead.
To provide the remarkably high capacity of the double-layered capacitor, the electrode material should display such characteristics as good electrical conduction, high specific surface, chemical and thermal durability. All of these are highly typical of carbon materials. In the recent years, the range of carbon nanomaterials promising for making electrochemically active electrodes has widened because of the mono-layered and multilayered nanotubes. Carbonic nanotubes exceed traditional materials in some parameters. A special interest is evoked by the geometry in which the array of carbonic nanotubes is predominantly perpendicular to the surface of the conducting substrate, which results both in a great increase in the effective surface of electrodes and in the improvement of the conditions of electric current flow.
Today, the scientists from the Institute of Inorganic Chemistry SB RAS have developed the methods for synthesis of arrays of carbon nanotubes up to 3 mm long. The thickest array was made as a result of continuous injection of the mixture of hydrocarbon and a catalyst at 800° C. Specific capacity of the supercapacitors from the arrays of directional carbonic nanotubes in aqueous electrolytes is 100—120 Farad/g.
Capacity can be increased still further by applying on the nanotube surface a substance that is able to change its structure reversibly as a result of chemical reaction affected by current. This electrochemical element is not a true supercapacitor but practically an accumulator. When it is discharged, the chemical energy accumulated in it is transformed in current.
There are a number of polymers, which can be used as a structure with good redox characteristics. Scientists from the Laboratory of Physicochemistry of Nanomaterials of the Institute of Inorganic Chemistry apply a thin layer of polyanilin for modifying the surface of the nanotubes grown on silicon plates. The best samples feature a layer of polyanilin 10 nm thin, which is comparable to the average radius of the nanotubes themselves. This thickness of polymer provides perfect conditions for current collection, and current density reaches the values comparable to currents in traditional ionistors. And the specific electrolytic capacity of the developed composite materials is much higher and reaches 500 Farad/g. These composites endure a large number of recharging cycles.
An important stage of the research conducted was studying the interaction of nanotubes and polyanilin. The methods of electron microscopy, X-ray diffractometry, infrared and X-ray electron spectroscopy have revealed fundamental regularities of transfer of electric charge in the layer between polymer and carbon. The obtained results are important for developing prototypes of electrochemical accumulators of electric energy for the auto- and aircraft industry, and for household electric appliances.