Fluorographene: The Modern History of Old Materials
The research team of A. Geim in collaboration with the scientists from the Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, has synthesized a new two-dimensional graphene compound, fluorographene. This compound was synthesized using the methodologies elaborated at the Institute of Inorganic Chemistry many years ago
The end of 2010 was marked with an event memorable for the researchers dealing with carbon materials: our former fellow countrymen A. Geim and K. Novoselov, currently working with the University of Manchester (United Kingdom), were awarded the Nobel Prize in Physics “for groundbreaking experiments regarding the two-dimensional material graphene”.
Graphene, which is produced by a mechanical separation of a monolayer from the surface of a graphite crystal, is a planar polymeric structure of carbon atoms. This structure displays a high strength and, most importantly, is stable on a silicon substrate. Owing to a high mobility of charge carriers in graphene (their velocity is close to that of light; Geim and Novoselov, 2007), this material may in the future replace silicon, the basis of modern microelectronics.
Nonetheless, there are some hindrances on this way - first and foremost, the problem of large scale production of graphene. The second problem is that graphene lacks the so-called forbidden zone, i.e., the energy gap between the valence band and conduction band, which makes less controllable the transition between the conductive and nonconductive states, necessary for binary logic operations. This problem can be solved with the help of a chemical modification (doping) of graphene, making it a semiconductor of either p- or n-type depending on the properties of the functional groups or atoms attached to carbon.
Two modified two-dimensional chemical compounds of graphene have been synthesized so far. The treatment of the initial layer with hydrogen plasma gave hydrogenated graphene, or graphane (Elias et al., 2009). However, this material loses its stability at a temperature slightly exceeding the room temperature. Another graphene modification was obtained by the research team headed by A. Geim in collaboration with scientists from the Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences (Novosibirsk, Russia). Xenon difluoride acting on graphene allowed the fluorinated graphene, or fluorographene, to be synthesized (Nair et al., 2010).
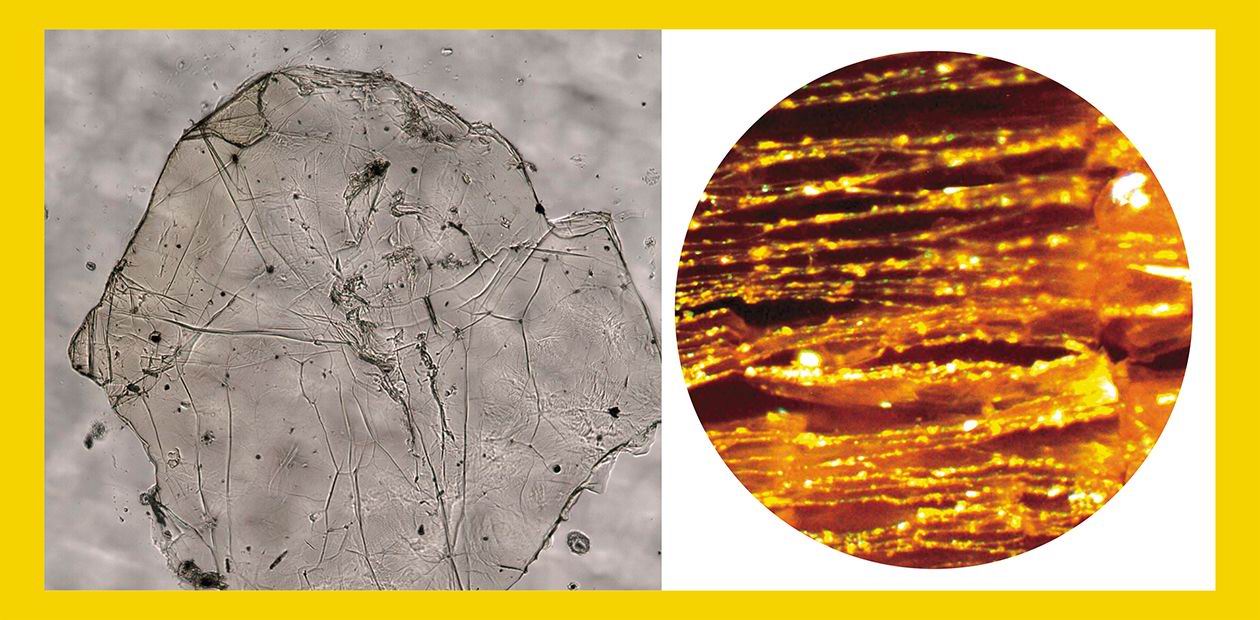
In its mechanical properties, the new material is comparable to graphene. Moreover, it is resistant to heating and to the impact of several liquids, and has a reasonable width of the energy gap, about 3 eV. Fluorographene may be regarded as a two-dimensional analog of another polymeric compound of carbon and fluorine—polytetrafluoroethylene (CF2)n, better known as Teflon.
When fluorinating graphene on a silicon support, involved in the reaction is not only carbon, but also silicon, which contaminates the target product. Therefore, the silicon support, traditionally used for producing graphene layers, preliminarily had to undergo special procedures. However, there is another, simpler, method for producing fluorographenes by successive separation of layers from a crystal of graphite fluoride. A similar approach has been recently used when sonicating one of the graphite fluorides with a stoichiometric composition (CF)n in sulfolane (Zboril et al., 2010).
There is one more graphite fluoride (C2F)n, where only half of the carbon atoms is bound to fluorine. The Institute of Inorganic Chemistry has been involved in the synthesis and study of this material for over 30 years. Relatively safe protocols for producing graphite fluoride with the potential to be upscaled to a commercial level have been proposed; this approach implies initial bromine intercalation into graphite. The inserted large bromine molecules as if “drive apart” the graphite layers, enhancing the penetration of fluorinating agent into the interlayer space (Nikonorov and Gornostaev, 1979; Yudanov and Chernyavskii, 1987).
The graphite fluoride compound thus produced always contained a certain amount of bromine and fluorinating agent as “guest” molecules. It has been found that these molecules can be replaced with other organic or organometallic compounds. This method is actively studied and even finds some applications.
In particular, successively increasing the size of a “guest” molecule, they succeeded in increasing the distance between graphite semi-fluoride layers to 1.2 nm, which exceeds approximately 3.5 times the interlayer distance in graphite. This gives the possibility to separate the layers either mechanically or with the help of a solvent, thereby eventually obtaining a “stack” of semi-fluorinated graphenes.
A three-dimensional semi-fluorinated graphite may display the properties that are not less interesting. For example, a “hot” current issue is the search for magnetism in the carbon-containing materials, and several specimens of the substance in question display a magnetic ordering at a temperature of ~5 K (Makarova et al., 2012). According to the preliminary data, the conditions for transition from a paramagnetic to ferromagnetic behavior are determined by the length of the carbon chain that remains non-fluorinated.
In addition, the methods for chemical removal of fluorine from the surface of a graphite fluoride layer have been elaborated at the Institute of Inorganic Chemistry; as a result, a carbon layer with a p-type conductivity is formed on the surface of this insulating material. Such material is an effective sensor for many gases, in particular, ammonia (Okotrub et al., 2009, 2010). The main advantage of such sensor is in that the regeneration of its initial state requires only a short exposure of the surface to an air flow.
Thus, similarly to the history of mankind, the chemistry of carbon structures follows a spiral pattern, revealing the new potential and prospects of old, well-known, and seemingly comprehensively studied materials and methods. Or their time just comes again?
L. G. Bulusheva, Doctor of Chemistry, and Prof. A. V. Okotrub, Doctor of Physics and Mathematics (Institute of Inorganic Chemistry, Siberian Branch, Russian Academy of Sciences, Novosibirsk, Russia)
References
Nikonorov Ju. I., Gornostaev L. L. Issledovanie vzaimodejstvija grafita s zhidkim triftoridom broma // Izv. SO AN SSSR. Ser. him. nauk. 1979. Vyp. 9. S. 55—59.
Judanov N. F., Chernjavskij L. I. Model’ stroenija interkalirovannyh soedinenij na osnove ftorida grafita // Zhurn. strukturn. himii. 1987. T. 28. S. 86—95.
Nair R. R. et al. Fluorographene: A two-dimensional counterpart of Teflon // Small. 2010. Vol. 6. P. 2877—2884.