Ruled by the tumor: why are metastases so hard to suppress?
A single mutated cell left undestroyed among trillions of normal cells in the human body triggers a cascade of events that result in a growing tumor. As the disease progresses, some cancer cells form metastases – secondary sites of tumor growth. Very often even timely removal of the primary tumor and post-surgery therapy do not lead to remission. It turns out that the primary tumor is capable of “tutoring” the microenvironment in the future locations of its metastases on the earliest stages of its development; metastatic cells also adjust their gene expression in a way that gives them a chance to settle in in new locations. If we learn how to prevent the growth and development of metastases, we will be able to save lives of up to 90% of all people dying from common types of cancer
In the past few decades, oncologists have been focusing their efforts on scrutinizing the mechanisms behind the origins of tumors, regulation of their active growth and formation of a favorable environment by the stroma, which is the scaffolding of organs primarily made up of connective tissue. Eventually, it became clear that the main danger of cancer lies in its ability to spread across the body.
Some cancer cells of the primary tumor leave it and enter the bloodstream or the lymph flow, traveling along the main routes of the vascular system. Cells that make it to the end of the path enter the stroma, due to being captured in the capillaries and adhering to the internal wall of the vessels. Only a small part of these cells survives in the new environment, but these cells can give rise to the new tumor growth. The process of dissemination of cancer cells across the body, called metastasis (Greek μετάστασις, metastasis, meaning “removal”, “change”) was first described in 1889 by James Paget, a British surgeon and pathologist; however, its mechanism long remained a mystery to the scientific community.
Paget drew an analogy between metastases and germination of seeds, which also survive only if the conditions of the soil – the microenvironment – are favorable. Back then, it was impossible to prove this experimentally, and for a long time, the dominating theory was the one proposed by James Ewing, an American pathologist, which stated that the main role in the dissemination of metastases in the body is played by bloodstream dynamics and structure of the vascular system.
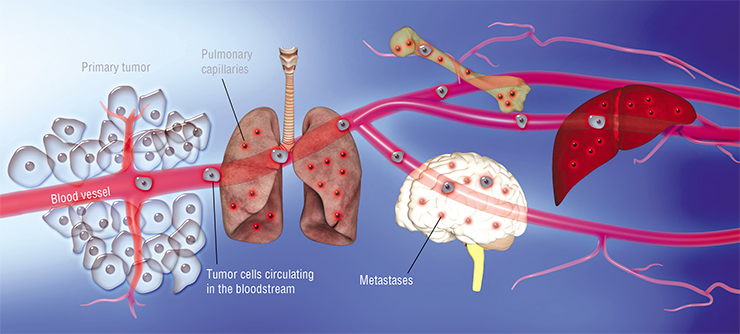
At last, in the 1970’s, in a series of experiments in mice injected by isotope labeled cancer cells, Isaiah Fidler, was able to prove that metastasis is influenced by the nature of specific cancer cells: cells of melanoma metastasized into the lungs, but not the liver, where they did not survive in the blood vessels. Later, a number of other factors were discovered, proving that different cancer cells tend to metastasize predominantly into specific organs, and often in predictable order. For instance, breast cancer cells first form metastases in the bones, liver, lymph nodes, and lungs, and only then enter the brain. Such specific distribution of metastases across the body is called organotropy.
Today’s cancer biology is still is in process of answering questions: what is a difference between metastasizing (disseminating) cells and other cells of the primary tumor? What drives the organotropy of metastatic cells? And, most importantly, how do metastases survive after the removal of the primary tumor and chemotherapy?
Metastases: from genetics to epigenetics
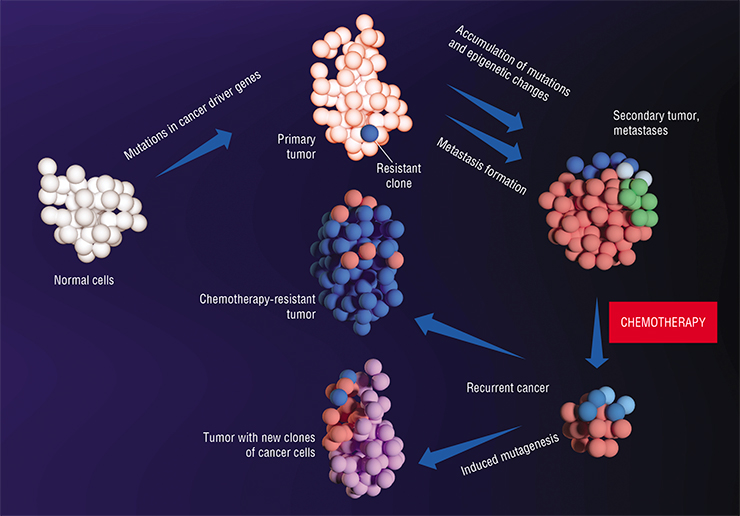
Cellular behavior is genetically predefined. Malignant transformation of normal cells into cancer cells is defined by mutations in the driver genes, which cause uncontrolled cell division. These mutations are accompanied by mutations in other genes, which initially have no impact on tumor growth – the so-called passenger genes. With each new generation of cells, with every new clone, these genetic changes accumulate. Some cell clones are more successful than others – this is why we can speak of “evolutionary” changes within the tumor.
Metastases are like germinating seeds: both survive only if the “soil” is right. They differ in the fact that the right conditions for their formation are created by the primary tumor itselfOne hypothesis states that cells acquire the ability to metastasize after similar accumulation of mutations in their driver genes launching this process. However, this hypothesis was experimentally disapproved: today, scientists generally agree that the majority of the primary tumor cells have the capacity for metastasizing. A genetic study of pancreatic cancer cells that disseminated into lymph nodes, liver, and lungs, showed that the founder cells of metastases had the same profile of driver mutations as the primary tumor: their genetic similarity is even higher than in randomly picked cells of normal tissue (Alderton et al, 2017).
What happens to the genomes of metastatic cells later? Comparison of cells of primary breast cancer tumors, their local (i.e. located in adjacent lymph nodes) and distant metastases demonstrated that in these new sites, the genomes of cancer cells continue to evolve independently from the primary tumor (Yates et al., 2017). The profile of mutations in metastatic sites within an organ turns out to be similar, but it differs from organ to organ. This means that cancer cells adapt to their new microenvironment depending on the niche they occupy. Consequently, while initially cells of the primary tumor and its metastases had similar mutations, adaptation to the new environment initiated the emergence of new genetic changes. These mutations turned generally to be connected with passenger genes, not with driver ones.
Interestingly, there is at least one identified gene – FBXW7, mutations in which counteract the metastatic process (Mlecnik et al., 2016). This is due to the enhancement of pro-inflammatory reaction, increase in number and activity of T-cell lymphocytes, stimulating immune reactions against cancer cells. Hence the emergence of mutations in FBXW7 gene of cancer cells prevents the development of immunosuppression, usually supporting tumor growth.
When pre-metastatic niches with a favorable environment are formed, some of the cancer cells leave the primary tumor and form micro-metastases in other tissues and organs. The final stage of forming a secondary tumor can take from several months to several yearsUntil now, we were talking about the changes in the genetic code, DNA. The next level of gene expression regulation is epigenetic – such as changes in methylation of DNA and histones (DNA-binding proteins). By their nature, these changes are more plastic compared to genetic changes: they are influenced by cell signaling cascades usually initiated by external factors.
It turned out that in metastatic cells, the “epigenetic code” is very different from that of the primary tumor. For example, in pancreatic cancer, metastases have a significant decrease of histone and DNA methylation. As the result, regions of inactive chromatin (the substance chromosomes are made of) switch into active state, becoming accessible to transcription factors which control the transcription of DNA into messenger RNA. This mechanism enhances the expression of oncogenes in the metastatic cells (Alderton, 2017).
At the crossroads of metabolic pathways
Epigenetic regulation is one of the key mechanisms of cell metabolism programming. As the tumor grows, it develops parts that lack blood vessels, resulting in a decreased supply of oxygen (hypoxia). Demethylation of DNA in cancer cells causes increased activity of the gene coding the HIF-1-alpha protein, called hypoxia-induced factor. Increased production of this protein, in its turn, affects the levels of expression of genes of many metabolic enzymes and transporter proteins, causing complex changes in the metabolism of the cancer cells.
Cellular metabolism is known to include processes of breakdown of compounds that release energy (catabolism), and their formation, which requires energy (anabolism). The energy metabolism of the cell works thanks to cell respiration, which includes glycolysis (enzyme-driven breakdown of glucose), tricarboxylic acid cycle (oxidative transformations of intermediate products of decay and synthesis of proteins, fats, and carbohydrates), and oxidative phosphorylation (storing of energy as the result of oxidation of organic molecules).
Cancer cells adapt their energy metabolism to oxygen-deficient environment in different ways. In the primary tumor, they rely predominantly on anaerobic glycolysis and not on oxidative phosphorylation, like normal cells do. Such increased uptake and breakdown of glucose to lactate, which is secreted by cancer cells, has been called the Warburg effect. This adaptation allows cancer cells successfully survive and proliferate in oxygen-deprived conditions.
However, this is true about the primary tumor; as for metastatic cells, their metabolic adaptations are still poorly understood. By using breast cancer cells, which have a broad range of metastatic organotropy, scientists discovered differences in the metabolisms of the primary tumor and its metastases. Cancer cells that colonized bones and lungs used oxidative phosphorylation more actively, while those that colonized the liver relied primarily on glycolysis. Those cells that colonized all target organs, activated both metabolic pathways (Rosen, Jordan, 2009). Hence, metabolic flexibility seem to help cancer cells in colonizing new niches.
Why would metastases in different cases benefit from using different types of metabolism? The question remains unanswered.
Preparing the “soil” for metastases
Despite their adaptive plasticity, metastatic cells cannot cope with the challenge of settling in a completely new environment on their own.
In 2005, experiments by a group of scientists led by David Lyden demonstrated that the primary tumor stimulates the formation of the so-called pre-metastatic niches in different organs. This happens partly due to stimulation of the vascular endothelium growth factor receptor (VEGFR-1) on myeloid progenitor cells (precursors of different types of blood cells – platelets, granulocytes, monocytes, and thrombocytes) in the bone marrow, which triggers their migration to the metastatic sites. Secondly, fibroblasts (cells of the connective tissue) located in these sites increase production of fibronectin – a glycoprotein component of the extracellular matrix. Myeloid precursor cells have receptors for cellular adhesion to this protein and literally “take the bait” and populate the pre-metastatic niches, where they start secreting inflammatory cytokines, growth factors and pro-angiogenic factors that stimulate blood vessel formation. Together, these factors facilitate changes in the organ stroma and its colonization by metastases.
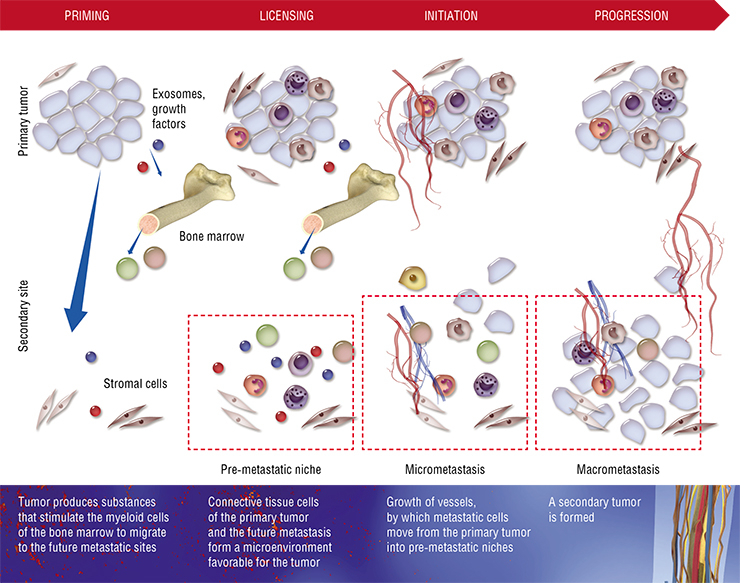
Even though the scheme described above is typical for the majority of organs, there are organ-specific aspects. For instance, in the liver and lungs, other blood cells actively participates in the creation of pre-metastatic niches – the neutrophils, a major type of granulocytes. We know that leukocytes of this kind help cancer cells to integrate in their new niche by secreting proteases and cytokines, as well as by direct contact with cancer cells when they leave capillaries.
Metastases in bones are especially treacherous and their pre-metastatic niche has special features. For instance, in breast cancer, cells lacking estrogen receptors can metastasize to the bone. Such cells in the primary tumor actively produce an enzyme called lysyl oxidase. In the bones, this enzyme stimulates the formation of osteoclasts – giant macrophage cells that resorb bone tissue (Cox et al., 2015). The cavities formed by the destructive action of osteoclasts are eventually filled by metastases.
The main function of lysyl oxidase is to catalyse the cross-linking of collagen fibers in connective tissue. Hypoxia causes breast cancer cells to increase the production of this enzyme, which facilitates the remodeling of the extracellular matrix during the preparation of yet another pre-metastatic niche – in the lungs.
Some cancers, such as melanoma, need new lymphatic vessels formed in the pre-metastatic niche to successfully metastasize into lymph nodes and distant organ sites. Recent research has shown that such lymphangiogenesis begins at early stages of the primary tumor growth and is mediated by midkine, a growth factor secreted by the melanoma cells (Olmeda et al., 2017).
These differences in specific mechanisms of niche formation may be one of the possible explanations of organotropic metastasis. But how do cancer cells, which have potential to metastasize into a number of organs, choose a particular one? The key known mechanism here is “educating” the future organ site with the help of exosomes – microscopic extracellular vesicles secreted by cells. The lipid membrane surface of exosomes carries receptors, and inside there are RNA and proteins.
Scientists carried out an interesting experiment: they extracted exosomes from cancer cells of different origin (breast cancer, pancreatic cancer, etc.) and injected them into the bloodstream of laboratory mice that had been implanted with other types of tumors (Hoshino et al., 2015). It turned out that exosomes could be used to reprogram the distribution of metastases across the body.
What stands behind this process? Exosomes from different cancer types carry receptors to specific proteins of the extracellular matrix on their surface, acting like a mail service: they are delivered specifically to the organ in which the stroma contains a lot of this particular protein. By merging with the stromal cell membranes, exosomes release their contents. This triggers pre-metastatic transformation in the cells: in lung fibroblasts, it works through activation of S100 group of genes; in Kupfer cells of the liver – of other genes of the same group. As the result, a series of cell signaling cascades and inflammatory reactions are initiated, “educating” the pre-metastatic niche (Hoshino et al., 2015).
All in all, the metastatic mechanisms discussed here certainly reflect the complexity of oncological diseases. The heterogeneity of the primary tumor and its metastases requires special attention in the development of treatments and highlights the need in combined and targeted treatments at different stages of the disease. This is in line with the results of the recent study assessing treatment of a patient with recurrent cancer between extended immunotherapy courses (Jimnez-Snchez et al., 2017). The comparison of T lymphocytes populations from the microenvironment of different metastases showed that they are heterogeneous. Therefore, primary, secondary and consecutive tumors respond to treatment differently.
We now know that cytostatic drugs cause cancer cell subclones to increase the secretion of growth factors and launch cell signal cascades that prevent cell death. Moreover, their survival is facilitated by the support from stromal cells, which switch to “defensive” behavior under the influence of chemotherapy. As the result, often cancer cell subclones with mutations providing resistance to the used drugs survive chemotherapy.
The discovery of pre-metastatic niches and understanding of their structure and function opened a new avenue for treating oncological diseases. If we find more ways to prevent or supress this process of preparing “fertile soil” for metastases, the chances of remission would increase many-fold.
References
Alderton G. K. Tumour evolution: epigenetic and genetic heterogeneity in metastases // Nat Rev Cancer. 2017. V. 17. N. 2. P. 141.
Cox T. R., Rumney R. M. H., Schoof E. M., et al. The hypoxic cancer secretome induces premetastatic bone lesions through lysyl oxidase // Nature. 2015. V. 522. N. 7554. P. 106–110.
Jimnez-Sanchez A., Memon D., Pourpe S., et al. Heterogeneous Tumor-Immune Microenvironments among Differentially Growing Metastases in an Ovarian Cancer Patient // Cell. 2017. V. 170. N. 5. P. 927–938.e20.
Hoshino A., Costa-Silva B., Shen T.-L., et al. Tumour exosome integrins determine organotropic metastases // Nature. 2015. V. 527 N. 7578. P. 329–335.
Mlecnik B., Bindea G., Kirilovsky A., et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastases // Sci Transl Med. 2016. V. 8. N. 327. P. 327ra26-327ra26.
Olmeda D., Cerezo-Wallis D., Riveiro-Falkenbach E., et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine // Nature. 2017. V. 546 N. 7660. P. 676—680.
Rosen J. M., Jordan C. T. The increasing complexity of the cancer stem cell paradigm // Science. 2009. V. 324. N. 5935. P. 1670–1673.
Yates L. R., Knappskog S., Wedge D., et al. Genomic Evolution of Breast Cancer Metastases and Relapse // Cancer Cell. 2017. V. 32. N. 2. P. 169–184.e7.
This publication is based on an article presented at the popular science contest “Bio/mol/text”-2017 on the Biomoleсula website (biomolecula.ru)