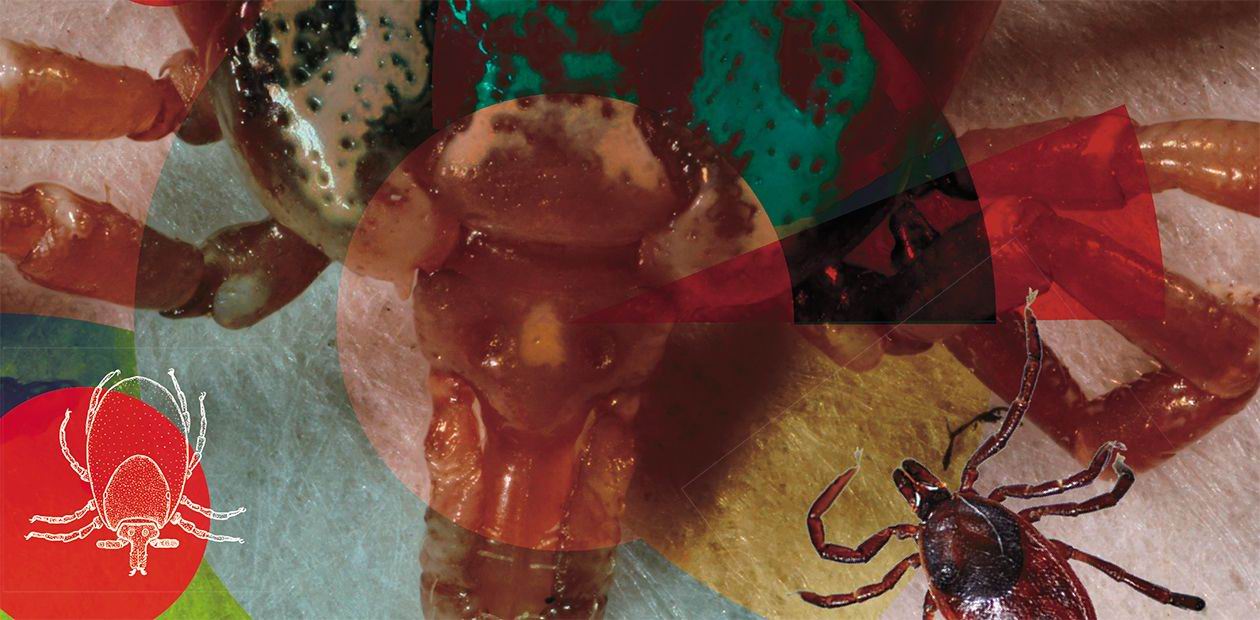Ticks That Bite Us
Every year, hundreds of thousands of people seek medical help after being bitten by ticks. For these blood-sucking parasites, humans are only incidental, casual prey; however, ticks carry an entire arsenal of pathogens of human and animal diseases. The problem of tick-borne infections concerns everyone who goes out into nature, including our pets; farm animals do suffer, too, from these infections. This article aims to better acquaint the reader with the habits and lifestyle of ixodid ticks, which occur most widely in Russia and can carry about three dozen diseases, including tick-borne encephalitis and Lyme disease. In recent years, the problem of tick-borne infections has exacerbated since the geographic range of these ticks has been expanding rapidly in Russia and worldwide
Ixodid ticks occur all over the world, on all the continents as well as islands and even the coasts of the Arctic and Antarctic, and in various climatic zones, from taiga to desert (Balashov, 1998). There are both omnivorous tick species and highly specialized ones, which feed only on birds, bats, or small mammals (Estrada-Pena et al., 2017).
The main harm for animal hosts comes from direct parasitization of many ticks on one animal, which can lead to a high blood loss or severe intoxication as a result of the parasite’s saliva entering the body. Cases are known of death of wild animals due to mass parasitization of ixodid ticks: wild antelopes in Zimbabwe National Park, young white-tailed deer in the United States, and moose in Canada and the northern United States.

In the absence of special anti-tick treatment, farm cattle may also host large quantities of ticks. Thus, several dozens of ticks can be feeding on one cow or sheep simultaneously and several hundreds, during one season; the total blood loss in the animal can be as high as several liters! Cases of death in sheep have even been described as a result of parasitization of several hundred ticks (Balashov, 1998).
Speaking about humans and their pets, such as dogs, ticks pose danger to them mainly because of a high risk of infection even after a single bite. Ixodid ticks transmit pathogens of viral, bacterial, and protozoal infections that can be contracted by humans. It is impossible to eradicate these diseases because their foci exist in nature independently of humans, and the carriers themselves are an integral part of natural ecosystems. Thus, in order to protect against ticks, you need to know the behavior and ecology of our potential “enemy.”
And here comes the ixodid tick
Many ixodid ticks have only photosensitive cells instead of eyes, so they “hunt” using other sensory organs.
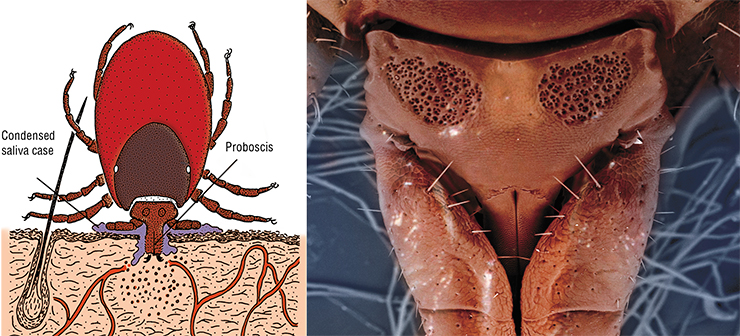
To detect a potential victim at a long distance, ticks rely mainly on their multifunctional Haller’s organ, which enables them to perceive changes in the carbon dioxide concentration, specific odor components associated with a potential host (hydrogen sulfide, ammonia, etc.), and thermal radiation, at a distance of up to 10 m. Having reached the victim, ticks determine the best place for suction using their sensilla, very sensitive organs consisting of a cuticular hair and receptor cells, which occur in especially large numbers on their legs and mouth apparatus.
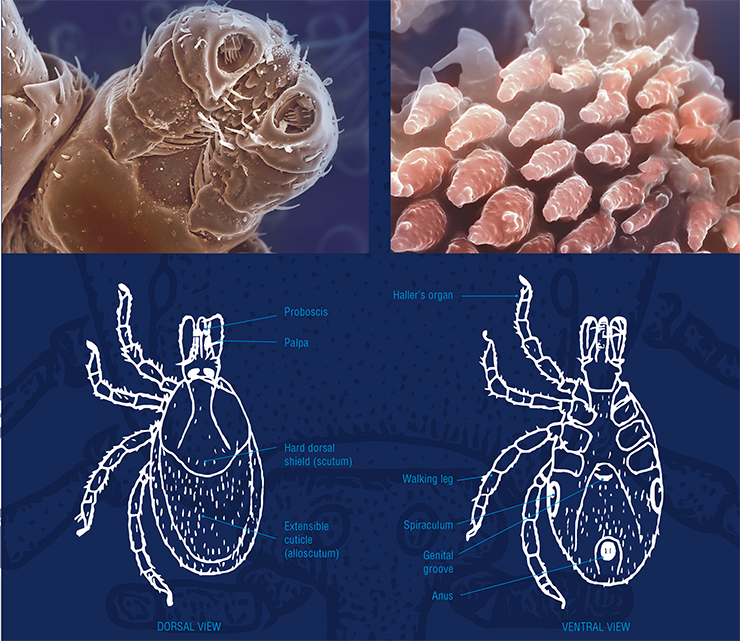
The family of ixodid ticks, Ixodidae, comprises six main genera: Ixodes (249 species), Haemaphysalis (166), Amblyomma (142), Rhipicephalus (79), Dermacentor (36), and Hyalomma (25 species). The Latin name for each tick consists of two parts that indicate the genus and species, and well-studied species also have a name in the language spoken in the country, e. g., the taiga tick (Ixodes persulcatus) and meadow tick (Dermacentor reticulatus).
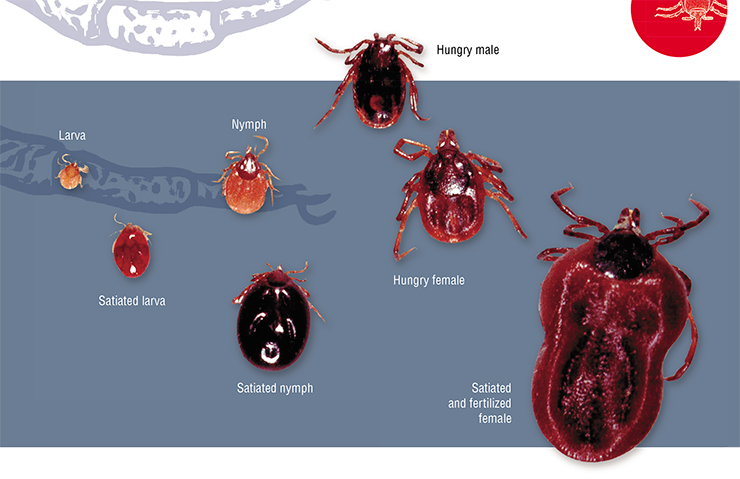
Ixodid ticks have the largest size of all: adult ticks can be as large as 2–13 mm in length. The taiga tick I. persulcatus, the most widespread one in Russia, has the following body length (for hungry individuals): female, 3–4 mm; male, 2–3 mm; nymph, 1.2–1.7 mm; larva, less than 1 mm. The size of satiated ticks is much larger
The tick’s paired salivary glands release secret containing many biologically active components, which act as anesthetics, interfere with blood coagulation, suppress the host’s immune responses, stimulate the release of histamine by the host’s cells, etc. These glands can also secrete components of the cement case involved in fixing the tick on the host’s body.
The salivary glands have another unique function, i. e., osmoregulation. If there is a risk of drying out, they secrete hygroscopic saliva into the tick’s preoral cavity to adsorb water molecules from the air, which allows the tick to retain the necessary moisture and stay alive for many months between blood suctions. And vice versa, when sucking blood, the tick is able to return about 70 % of excess water and salts through salivation at the bite site. As a result, the volume of saliva secreted by a tick during the entire feeding period significantly exceeds the body mass of a satiated tick (Biology of Ticks, 2014).
The body of an ixodid tick consists of two main parts: an unsegmented body with legs (six in larvae and eight in nymphs and adult males and females) and a capitulum (head). On the dorsal side, ticks have a hard shield, which covers, in females, nymphs, and larvae, only the anterior third or half of the body. The rest is covered with an extensible cuticle, which forms in hungry individuals a system of parallel microfolds that expand during feedingMost ixodid ticks sit in ambush, waiting for their prey (ambush-type parasitism). Hungry ticks climb up plants, where they sit and wait for potential hosts to pass by. When a tick receives the necessary signal, it goes into a state of active waiting, whereby it makes vibrational movements with its outstretched first pair of legs until it comes into direct contact with the host animal.
Many ticks of the Ixodes genus exhibit a nest–burrow type of parasitism, whereby hungry individuals, from larvae to adults, attack potential hosts in burrows and nests; some ticks have a mixed type of parasitism. There are ticks adapted for living in people’s homes, e. g., the brown dog tick Rhipicephalus sanguineus (Balashov, 1998; Yakimenko, 2013; Biology of Ticks, 2014).
Both food and home
 Once a tick attaches itself to a host, it begins vigorously searching for a convenient place for suction, preferably where it is more difficult for the host to get rid of the parasite, e. g., on the ears or head of rodents. Ticks spread more evenly on hedgehogs because the animals cannot remove the parasites hidden between the needles. In regions with a dry and hot climate, ticks often choose for suction the sun-protected lower part of the body. One can even find ticks in the oral cavity of elephants and in the nostrils of hippos and camels.
Once a tick attaches itself to a host, it begins vigorously searching for a convenient place for suction, preferably where it is more difficult for the host to get rid of the parasite, e. g., on the ears or head of rodents. Ticks spread more evenly on hedgehogs because the animals cannot remove the parasites hidden between the needles. In regions with a dry and hot climate, ticks often choose for suction the sun-protected lower part of the body. One can even find ticks in the oral cavity of elephants and in the nostrils of hippos and camels.
Ticks bite people in various places: in the armpits; less often on the chest, belly, arms, buttocks, and legs; and also on the head, especially in children.
A tick attaches itself to the host’s skin with its mouth organs; cuts through the skin; and sucks out with its proboscis the host’s blood, inflammatory infiltrate, and tissue dissolution products, alternating this process with injecting saliva into the wound.
Ixodid ticks feed slowly: larvae feed for 3–5 days; adult females, for 5–15 days. During feeding, their body mass increases by one or two orders of magnitude: females of the most massive species can absorb up to 8–10 ml of blood!
After feeding, larvae and nymphs fall off the host and molt, moving to the next stage in their development. In adult ticks, feeding is associated with reproduction. Partners meet each other, as a rule, on their host—hungry males and females cannot mate, with the exception of ticks of the Ixodes genus. A male tick can stay on its host for several months and fertilize, during this time, several dozens of females. A female tick mates only once, after which it quickly gains mass, falls off the host and after some time (from several days to several months) lays eggs (from 800 to 20,000 depending on the species), after which it dies (Balashov, 1998; Biology of Ticks, 2014).
In most cases, ixodid ticks develop with a change of their host at each stage in their life cycle. Ticks’ hosts include birds, reptiles, and, primarily, mammals, especially rodents. Thus, the taiga tick parasitizes on about 170 species of birds, 100 species of mammals, and several species of reptiles. As a rule, small animals moving in the lower tiers are attacked by larvae and nymphs, and large animals are a target of adult ticks (Balashov, 1998; Biology of Ticks, 2014).
Expanding the borders
Over the past decades, the range of ixodid ticks has changed and expanded. Thus, if earlier it was believed that in Russia, the problem of tick-borne infections is relevant only for Siberia and the Far East, now it has become a concern for people living in the western part of the country. Ticks are also spreading in other regions of the planet: the United States, Canada, China, Southeast Asia, and South America.
Scientists name climate warming as one of the causes of this phenomenon. It has been repeatedly shown that migratory birds can carry ticks over long distances (Sparagano et al., 2015). Previously, ticks were unable to survive in the north, but now the situation has changed. In many regions of the planet, winters have become shorter and milder, making it easier for ticks to overwinter.

Thus, in Sweden and Norway over the period 1994–2008, the border of the tick’s geographic range moved by more than 200 km northward along the Baltic coast (Jaenson et al., 2012). On the North American continent, ticks began to appear almost 1000 km to the north compared with 1943—1983. In the mountains in the north of the Czech Republic, where the temperature has risen by 1.4 °C over the four decades, ticks have appeared at an altitude of 1300 m above sea level. In Russia in the 1960s, the taiga tick was found only in the southern regions of the Komi Republic, but now it also inhabits the central regions—over the past forty years, the northern border of its range has moved by 150–200 km (Loktev, 2015).
The second possible cause behind the change in the tick’s range is a change in ecology due to human activity. For example, naturalists who traveled to the United States in the middle of the 18th century noted a large number of ticks. But a century later, due to the development of agriculture, the number of white-tailed deer, the main host of blacklegged ticks, dropped sharply, leading to a decrease in the quantity of these parasites. However, in the second half of the last century, the number of both host animals and ticks increased sharply. Consequently, the number of tick attacks on humans also increased as townspeople began to spend more time in nature.
In Western Siberia, the tick Ixodes pavlovskyi has recently expanded its range considerably northward, and the cause of this event remains unclear. At the end of the last century, its range was still confined to Altai and other mountainous regions (the Salair ridge and Kuznetsk Alatau). Now it occurs on most of the explored plains of Novosibirsk and Tomsk oblasts; moreover, in some areas, mainly those with high anthropogenic loading, I. pavlovskyi almost completely replaced the taiga tick (Livanova et al., 2011; Romanenko, 2011).
This change in the tick’s range may be due to the many years of repeated anti-tick treatments around large cities, which were carried out in the 1960–1980s. When ticks began to recolonize these areas, I. pavlovskyi gained an advantage because of a shorter life cycle and ability to feed on birds, which are greater in number in anthropogenic foci than large mammals, the main hosts of the taiga tick.
In some cases, the tick’s range expands southward. Thus, in Western Siberia in the 1960–1970s, the range of the meadow tick shifted to the south, from the forest zone to forest–steppe. This event could have been caused by several factors acting simultaneously: acaricidal treatments of forests and changes in the composition of rodent populations, the main hosts of the meadow tick (Yakimenko, 2013).

The northward expansion of I. pavlovskyi population to the north coincided with another phenomenon, i. e., the discovery of its hybrids with the taiga tick. Interspecific hybridization presents a common phenomenon among closely related species of ixodid ticks. However, in the vast majority of cases, interspecific hybrids are not capable of reproduction.
Thus, in Russia and Estonia, in places where the geographic ranges of the taiga and castor bean ticks overlap, ticks were found with morphological and genetic characteristics of their hybrids (Bugmyrin et al., 2015). However, the offspring that came from laboratory crossbreeding experiments with these two species turned out to be nonfertile.
The entire geographic range of I. pavlovskyi is located within that of the taiga tick, and molecular genetic analysis identified ticks with a hybrid genotype in all the habitats of I. pavlovskyi in Tomsk and Novosibirsk oblasts and in the Altai Republic. In some places, the relative quantity of the hybrids exceeded 30 %, with both first and second generation hybrids being detected (Kovalev et al., 2015; Rar et al., 2019). All this suggests that these two tick species are capable not only of crossbreeding but also of producing fertile offspring
Simulations of the climate and biotope change as a result of human activity showed that in the 21st century, one can expect a contraction of the range of the taiga tick in countries bordering on Russia from the west, and by the end of the century, this process will spread to European Russia (Yasyukevich, 2019). Presumably, in Moscow oblast as well as regions bordering on it from the west and northwest, the taiga tick will disappear. A similar process will occur in areas lying to the south, where forest will be retreating due to climate warming, giving way to drier habitats.
Meanwhile, there are factors conducive for the taiga tick to expand its range considerably in Kamchatka krai and appear in Magadan oblast, and these predictions are beginning to come true. Thus, taiga ticks have recently been found in the neighborhoods of Magadan (Dokuchaev, 2015). On the Kamchatka Peninsula, these ticks have been observed for quite some time. Meanwhile, no larvae or nymphs have been found there, which would have served as indirect evidence that the taiga tick can go through its full life cycle in this region (according to the Center for Hygiene and Epidemiology in Kamchatka Krai, 2019). Perhaps, ticks have so far been brought there by migratory birds.
Tick infections attack
 The expansion of the geographic range of ixodid ticks has logically led to an increase in the natural foci of tick-borne infections. The number of infected ticks is growing everywhere—one can even encounter them in the parks of large cities.
The expansion of the geographic range of ixodid ticks has logically led to an increase in the natural foci of tick-borne infections. The number of infected ticks is growing everywhere—one can even encounter them in the parks of large cities.
The problem of tick-borne borreliosis has recently become a challenge for Japan, Turkey, and Korea. The level of infection of ixodid ticks in Belarus has increased sharply: over the last decade, the level of infection of the castor bean tick and meadow tick with the tick-borne encephalitis virus has increased manifold, and the share of castor bean ticks infected with the pathogen of tick-borne borreliosis has grown from 13 to 35 % (in some regions up to 60 %) (Bychkova et al., 2015).
In the last decade, the geographic range of tick-borne encephalitis has expanded in Russia. This process manifests itself most conspicuously in the Siberian Federal District—Krasnoyarsk krai, Novosibirsk and Omsk oblasts, and the Republics of Tyva and Khakassia, each of these regions now have one more district included in this range. For example, Novosibirsk oblast used to have 22 districts endemic for tick-borne encephalitis, but now their number has increased to 23 due to the adding of the Chany district.
The tick-borne encephalitis virus has not yet been detected on the Kamchatka Peninsula. However, in some areas, ticks were found to carry DNA of pathogens of tick-borne borreliosis, rickettsiae, and human granulocytic anaplasmosis (according to the Center for Hygiene and Epidemiology in Kamchatka Krai, 2019). Moreover, three areas in Kamchatka were recognized as endemic for tularemia, the pathogens of which can also be carried by ixodid ticks.
In Western Siberia, interspecific hybrids between the taiga tick and the tick Ixodes pavlovskyi were found to carry the same infectious agents as ticks of the parental species. As a result of different variants of crossbreeding, the genetic variability of ticks in a natural focus inhabited by both the parental species and their hybrids will be markedly higher than usual. This can lead to an increase in the tick activity period, expansion of the range of their potential hosts, and even an increase in the diversity (including genotypic) of infectious agents carried by the ticks. Since ticks with a hybrid genotype may be more adapted to different habitat conditions, one can expect further expansion of their range and, accordingly, the ranges of tick-borne pathogens.

There is another, subjective reason for the sharp increase in the number of reported tick bites and diseases. Previously, most people bitten by ticks threw them away and rarely sought medical help. Today, many people bring ticks for analysis and seek preventive treatment. All these cases are taken into account by medical statistics although, according to experts, today too these data are significantly underestimated.
All we can say today about tick-borne infections comes down to one main thing: the problem exists, and it is huge. Ticks are attacking, and there are more and more victims. Sadly, the changing ecological situation and human economic activity often turn out to be fatal for many useful arthropods, such as bumblebees and honeybees. But the blood-sucking ticks are doing all well. And they are bringing more and more trouble.
Ticks living in North America (United States) also carry a wide range of infectious agents, which cause tick-borne borreliosis (Lyme disease), human granulocytic anaplasmosis, human monocytic ehrlichiosis, babesiosis, Rocky Mountain spotted fever (the most severe form of rickettsiosis on the planet), etc. There is no viral tick-borne encephalitis in the United States, but the related Powassan virus occurs there in ticks. American tick species differ from Eurasian ones, with the exception of the brown dog and longhorned ticks, which occur on both continentsA prime example is the winter tick (Dermacentor albipictus), which parasitizes moose in Canada and the northern United States. The situation with this tick is becoming catastrophic—because of the climate warming, the ticks survive better in winter and become active earlier, thus having more time to find a victim. The winter ticks have always been a problem for moose, but there have never been so many of them. Covered with ticks, anemic animals rub themselves against trees so hard that they lose their fur—these animals are called “ghost moose.” When a moose calf is born, hungry ticks move from the mother to the newborn. More than half of the young animals die as a result of the mass invasion, with up to 100,000 ticks being found on the corpses. Biologists fear that moose may disappear altogether in the US Midwest (Balashov, 1998).

Ticks are not only expanding their traditional habitats; they are exploring completely new ones. Thus, in 2017, a rapidly growing population of previously unseen ticks was discovered in the US state of New Jersey; the newcomer was the Asian longhorned tick (Haemaphysalis longicornis), which usually occurs in Southeast Asia (China, Japan, and Australia). Now these ticks, which carry infections including tick-borne rickettsiosis, have spread to 17 states. The “migrants” from Asia are reproducing very quickly. The reason is that even an unfertilized female of this species can lay up to 2000 eggs in one clutch directly on the host animal. Breeding quickly, these ticks pose a real threat even to large cattle.

The currently observed expansion—in many regions worldwide—of the geographic ranges of different tick species, capable of carrying pathogens of infectious diseases in humans and agricultural and domestic animals, is a threatening phenomenon. It requires not only a thorough scientific study of the newly emerging natural foci of these diseases but also increased attention on the part of health systems and sanitary and epidemiological control agencies in different countries.
References
Balashov Yu. S. Iksodovye kleshchi – parazity i perenoschiki infektsii (Ixodid Ticks: Parasites and Carriers of Infections). St. Petersburg: Nauka, 1998 [in Russian].
Korenberg E. I., Pomelova V. G., Osin N. S. Prirodnoochagovye infektsii, peredayushchiesya iksodovymi kleshchami (Zoonotic Infections Transmitted by Ixodid Ticks), ed. by A. L. Gintsburg and V. N. Zlobin. Moscow: 2013 [in Russian].
Romanenko V. N. Mnogoletnyaya dinamika chislennosti i vidovogo sostava iksodovykh kleshchei (Ixodidae) na antropogenno narushennykh i estestvennykh territoriyakh (Multiyear trends in the quantity and species composition of Ixodid ticks (Ixodidae) in anthropogenically damaged and natural territories) // Parazitologiya. 2011. V. 45. N 5. P. 384–391 [in Russian].
Filippova N. A. Iksodovye kleshchi podsemeistva Ixodinae. Fauna SSSR. Paukoobraznye (Ixodid Ticks of the Subfamily Ixodinae. Fauna of the USSR. Arachnids). Leningrad: Nauka, 1977. V. 4. N 4 [in Russian].
Filippova N. A. Iksodovye kleshchi podsemeistva Amblyomminae. Fauna Rossii i sopredel’nykh stran Paukoobraznye (Ixodid Ticks of the Subfamily Amblyomminae. Fauna of Russia and Neighboring Countries. Arachnids). St. Petersburg: Nauka, 1997. V. 4. N. 5 [in Russian].
Yakimenko V. V., Mal’kova M. G., Shpynov S. N. Iksodovye kleshchi Zapadnoi Sibiri. Fauna, ekologiya, osnovnye metody issledovaniya (Ixodid Ticks of Western Siberia. Fauna, Ecology, Main Research Methods). Omsk: Omsk. Nauch. Vestnik, 2013 [in Russian].
Bugmyrin S. V., Belova O. A., Ieshko E. P., et al. Morphological differentiation of Ixodes persulcatus and I. ricinus hybrid larvae in experiment and under natural conditions // Ticks Tick Dis. 2015. V. 6. P. 129–133. https://doi.org/ 10.1016/j.ttbdis.2014.11.001.
Estrada-Pena E., Mihalca A. D., Petney T. N. Ticks of Europe and North Africa. A Guide to Species Identification // Springer. 2017. https://doi.org/10.1007/978-3-319-63760-0
Biology of ticks. Edited by Daniel E. Sonenshine and R. Michael Roe. Oxford University Press, 2014. 496 p.
Kovalev S. Y., Mikhaylishcheva M. S., Mukhacheva T. A. Natural hybridization of the ticks Ixodes persulcatus and Ixodes pavlovskyi in their sympatric populations in Western Siberia //Infect. Genet. Evol. 2015. V. 32. P. 388–395.
Rar V., Livanova N., Tkachev S., et al. Detection and genetic characterization of a wide range of infectious agents in Ixodes pavlovskyi ticks in Western Siberia, Russia // Parasit Vectors. 2017. V. 10(1). P. 258. doi: 10.1186/s13071-017-2186-5.
Rar V., Livanova N., Sabitova Y., et al. Ixodes persulcatus/pavlovskyi natural hybrids in Siberia: Occurrence in sympatric areas and infection by a wide range of tick-transmitted agents // Ticks Tick Borne Dis. 2019. V. 10(6):101254. doi: 10.1016/j.ttbdis.2019.05.020.