Behind the Scenes of Nobel Discoveries
Leonid Radushkevich: A Pioneer of Carbon Nanotubes
This publication was inspired by one of our authors – Dr. Alexander Petrov from the Voevodsky Institute of Chemical Kinetics and Combustion (Novosibirsk), who once told SCIENCE: First Hand an exciting story about the discovery of one carbon “nanomodification.” When carbon nanotubes hit the headlines in the early 1990s, following the sensational photographs by the Japanese physicist Sumio Iijima, Petrov remembered that he had previously heard of something alike. Soon after he indeed found in his old files an abstract of a paper by Soviet scientists L. V. Radushkevich and V. M. Lukyanovich, published in Zhurnal fizicheskoi khimii (Journal of Physical Chemistry) as early as in 1952, nearly four decades before Iijima’s famous work! Once we saw the striking photographs of carbon “worms,” which Soviet chemists took with a transmission electron microscope in the mid-twentieth century, we set out on a quest for information about the life and work of those who, in fact, hold the priority in the discovery of nanotubes. We managed to contact Leonid Radushkevich’s granddaughter Tatyana Golovenko. Her archives happened to contain unique photographs and an autobiography of this outstanding personality and reputable scientist, who nearly fell victim to the repressions of the 1930s because of his noble origin
Nowadays, the prefix nano- has become trite from excessive and often needless use yet its essence stays the same – when crossing the size threshold of 100 nm, a substance gains new physical properties in comparison with bulk materials. An excellent example of reduced dimensionality effects is carbon nanomaterials – from fullerenes, which are nanosized in all the three dimensions, to graphene molecules, which are essentially a single layer of graphite. In-between somewhere lie carbon nanotubes, which are nanosized in two dimensions only (Kats, 2008).
Nanotubes possess extraordinary mechanical, electrophysical, and physical properties, which render them useful in most advanced innovative technologies. Thus, depending on their structural and geometric characteristics, nanotubes can exhibit the properties of both metals and semiconductors. Moreover, their strength is much higher than that of the best steels yet their density is much lower, and they show very high resistance to deformation and high thermal conductivity. Mechanical engineering, computer technology, optics, electronics… Potential applications of carbon nanotubes are expanding as researchers are coming up with more in-depth studies of their properties and businesses are launching large-scale manufacture of this promising material of the future.

It is believed that the first carbon nanotubes were obtained as early as at the end of the 19th century. In 1889, the Americans T. V. Hughes and C. R. Chambers patented a technique of producing carbon filaments by pyrolysis of a mixture of methane and hydrogen and suggested using these filaments in incandescent lamps. However, Edison’s light bulbs, based on these filaments, had too short a service life (Varlamova, 2013). Even further into the past, the amazing properties of this material must have served to manufacture the famous Damascus steel. Evidently, this steel owes its unusual combination of firmness and flexibility to multilayer carbon nanotubes filled with cementite (iron carbide) (Reibold et al., 2006).
Nevertheless, the discovery of carbon nanotubes – the best known representatives of the fullerene family and the third form of pure carbon, after diamond and graphite – officially took place only in the twentieth century.
Shadowed by the Olympus of science
In 1991, the Japanese physicist Sumio Iijima discovered and described, using transmission electron microscopy, the geometry of amazing carbon structures that he had obtained by an arc discharge of atomized graphite. These nanotubes, as he called them, were essentially multi-walled hollow cylinders no more than a few nanometers in diameter, “rolled” from atomic carbon layers. Their structural description and microphotographs created a sensation in the scientific community and brought a real breakthrough in the research on synthesis and practical applications of the new material.
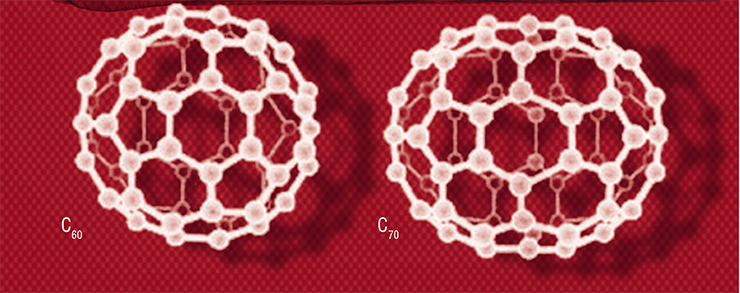
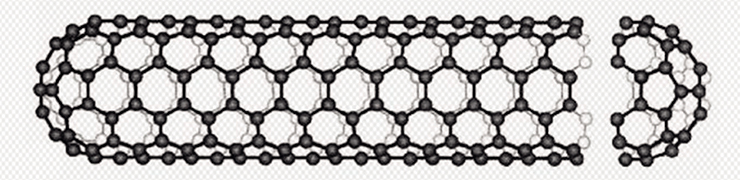
Fullerenes are polyhedral clusters of pure carbon whose molecular framework is built of pentagons and hexagons composed of carbon atoms. This family also includes nanotubes, i. e., extended cylindrical structures with a nanometer diameter and a length of up to several centimeters, which consist of one or more hexagonal graphite planes rolled into a tube
Iijima’s works saw the light six years after the sensational discovery of the very first known representative of the carbon nanomaterials family, i. e., Buckminsterfullerene, a C60 molecule shaped like a soccer ball. This molecule was first obtained in the lab as a result of laser irradiation of graphite while testing the hypothesis that carbon stars are the sources of complex carbon chains found in space. The experiment to simulate stellar conditions gave an unexpected result – the mass spectrometer showed a significant presence of C60 clusters in graphite vapor. While contemplating the stability of these huge molecules, one of the discoverers, British chemist Harry W. Kroto recalled the amazing Fuller’s Biosphere Dome, which served as a prototype for the US pavilion at the EXPO 67 World Exhibition in Montreal. This is how scientists came up with an assumption about the shape and structure of this compound, which was later named after the architect R. Buckminster Fuller.

In 1996, Kroto, together with the US scientists R. Curl and R. Smalley, won the Nobel Prize in Chemistry for the discovery of a fundamentally new form of carbon, and Iijima came close to winning the Nobel Prize in 2006–2007. However, these powerful achievements, which hit the very top of the Olympus of science, shadowed the prominent works of many other scientists, which either gained no further development or went unnoticed by the world scientific community. The results of these studies, many of which came ahead of their time, have now become the domain of the history of science.
 Trapped inside this “underwater part of the nano-isberg,” we find many Russian names. Thus, back in August 1985, a month before the publication on Buckminsterfullerene, a Kiev chemist M. Yu. Kornilov published an article “We Need Tubular Carbon,” where he predicted the possibility of existence of a new form of carbon, i. e., single-walled nanotubes. He came up with these ideas back in the late 1970s, but due to the complexity of the quantum – chemical calculations, he was unable to theoretically calculate these three-dimensional structures.
Trapped inside this “underwater part of the nano-isberg,” we find many Russian names. Thus, back in August 1985, a month before the publication on Buckminsterfullerene, a Kiev chemist M. Yu. Kornilov published an article “We Need Tubular Carbon,” where he predicted the possibility of existence of a new form of carbon, i. e., single-walled nanotubes. He came up with these ideas back in the late 1970s, but due to the complexity of the quantum – chemical calculations, he was unable to theoretically calculate these three-dimensional structures.
However, this kind of work was accomplished by a team of Moscow scientists from the Institute of Organoelement Compounds, USSR Academy of Sciences, who had been looking for closed hollow carbon structures where one could insert metal atoms. When it turned out that the C20 molecule, shaped as a dodecahedron, was unstable, I. V. Stankevich, one of the project participants and an avid soccer player, proposed a closed C60 structure with the symmetry of a truncated icosahedron, which was shaped, in fact, like a soccer ball. The quantum chemical calculation of this structure, carried out in 1971, showed that C60 was indeed a stable molecule. Nevertheless, chemists could not be persuaded to synthesize this structure, which is why, until 1985, it was considered a theoretical fantasy. However, to the credit of the Nobel laureate Smalley, he noted in his lecture the contribution of Soviet scientists to the discovery of fullerenes.
Going even deeper down the timeline, we arrive at the middle of the 20th century, namely, the year 1952, when Soviet scientists L. V. Radushkevich and V. M. Lukyanovich published in Zhurnal fizicheskoi khimii (Rus. Journal of Physical Chemistry) their paper “On the Structure of Carbon Formed during the Thermal Decomposition of Carbon Monoxide on an Iron Contact.” It included 12 photographs taken with a transmission electron microscope, which showed clusters of filamentary particles. They were 5–7 µm in length, and the thinnest ones about 30 nm in diameter. According to the authors, this “carbon has a very peculiar structure, hitherto not described by anyone… most of the particles have a characteristic worm-like shape with characteristic endings…, a channel passes inside the particles…, the particles themselves are hollow… with constant diameters along the entire length…”

LEONID RADUSHKEVICH
A prominent Russian physicist and chemist, doctor of chemical sciences, professor. Born on December 7, 1900 in Moscow. In 1928, he graduated from the Physics and Mathematics Department of Moscow State University. For many years he taught at the Military Academy of Chemical Defense.
He worked at the Institute of Physical Chemistry, Russian Academy of Sciences, from April 1948 until his death on November 9, 1972. Radushkevich is a coauthor of the well-known Dubinin–Radushkevich adsorption equation, which is applied worldwide. This equation was first published in 1947 and since then, it has played a key role in the development of the theory of volume filling of micropores. In 1997, the International Conference on Carbon (Carbon’97; United States) held a special meeting dedicated to the 50th anniversary of the Dubinin – Radushkevich equation.
Radushkevich’s main research interests dealt with capillary condensation in dispersed systems, the theory of kinetics and adsorption dynamics, the mechanisms of aerosol filtration processes, and the development of methods for studying aerosol filters and for studying the structure of adsorbents by electron microscopy. In 1952, he first obtained electron microscopic images of carbon nanotubes, and he also participated in the synthesis of those structures.
Radushkevich is a coauthor of about 100 research papers in leading Soviet scientific journals and the author of the textbooks A Course of Statistical Physics (1960) and A Course of Thermodynamics (1971). A participant in World War II, reserve colonel, awarded with the Order of the Red Star and the medal “For Victory over Germany.” Adapted from: (Voloshchuk, 2010)
The paper was received on January 5, 1951. Two years later, Nature published a brief communication by British scientists who also described “carbon worms” of a spiral shape yet could not figure out their internal structure. Nevertheless, the numerous papers published immediately after Iijima’s discovery completely overlooked those works by the British and Soviet scientists. As a matter of fact, fame never found them for what they did. However, in 2006, the journal CARBON published a joint article by its editor M. Monthioux and V. L. Kuznetsov from the Boreskov Institute of Catalysis (Novosibirsk), from which it followed that the priority in the discovery of multilayer nanotubes belongs to Radushkevich and Lukyanovich. Nowadays, references to their work can be found in academic dictionaries and in reviews on fullerenes.
Nano in the USSR
Who were they – the discoverers of carbon nanotubes? Unfortunately, we found very little information about Dr. V. M. Lukyanovich, not to mention his photographs. It is only known that in 1967, while studying the pulsed method of diamond growth, he and his colleagues from the Institute of Physical Chemistry became the first to obtain filamentary crystals, or the so-called diamond whiskers. These single crystals grow at low pressures yet with tremendous linear velocities. This event, listed in the USSR register of discoveries, was of great importance because it was the first-time successful attempt to obtain whiskers of a substance metastable under synthesis conditions. As for whiskers in general, they are highly promising materials because of their unique properties, including high strength and the ability to maintain elasticity at high temperatures.
We were much luckier with Leonid V. Radushkevich. Firstly, on the occasion of the 110th anniversary of his birth, A. M. Voloshchuk published his curriculum vitae, which we present here in full.

Fortunately, in addition to the desiccated phrases of this purely official document, we have an opportunity to draw upon the memories of Radushkevich’s granddaughter Tatyana Golovenko, as well as her personal archives, which contain Radushkevich’s childhood memoirs and biography, all written by the scientist himself. These materials draw a much clearer portrait of this complex personality, who spared no effort to work for his homeland but had an extremely negative attitude towards the Stalinist regime. That was not surprising – many of his relatives and friends died as a result of the repressions. The fate of Radushkevich himself hung in the balance because of his noble origin, which he chose to conceal, and he survived only by a miracle…
He never joined the communist party, despite his military and scientific status and the classified nature of his work, and such a decision was by no means common at that time. He never compromised on his values and was often straightforward and nondiplomatic in his speech. His daughter Zoya recalled that when Stalin died, her father crossed himself and said: “Thank God this damned tyrant is finally dead!” Later, a manuscript was found in his drawer, entitled eloquently Anti-Leniniana.
Below we publish excerpts from Radushkevich’s personal diary.
In first person: the beginning
Looking back at anyone’s life, there is always a lesson to learn, even in the life of Judushka Golovlev or Gobseck. Even more so in the life of a scientist. That is why I now find it in my heart to speak up. I have no time to go into details of my biography. I would only like to draw attention to some its accents and touch upon my own traits as a scientist.
“With love for art I came to birth; in childhood when the organ played high up in the age-old church of ours I’d listen with delight…” (Mozart and Salieri by Alexander Pushkin*). For me this organ instrument, once sounding so powerfully, was my father. An activist of 1905, a natural scientist by education, he graduated from Moscow University and left it for a provincial town, despite the offer of a chair position. He took passion in the ideas of Pisarev, Chernyshevsky, Darwin, Buckle, Timiryazev and longed for enlightenment work, as evidenced by his letters to my mother.

In Ryazan, where he moved, he began as a gymnasium teacher and then opened a private gymnasium himself, and became its first director. However, his main effort was to rally the local intellectuals, with whom he not only won trust but also gained some magical influence, which persisted for many years after his death… His main pursuit was to promote enlightenment, especially in natural sciences. His influence made possible the creation of new educational institutions, such as Yekimetskaya’s private gymnasium for girls. Many a person would stay influenced by him for decades.
What were the origins of that power? It had something to do with his father, my grandfather, a participant in the Polish uprising of 1863, who was sent to permanent exile in Siberia. But this rampant activity cost my father his health. He burned out fast, having contracted consumption at a young age, and died in 1908, leaving his wife with three small children. She quickly remarried, and the family grew by two more children, born in her second marriage.
Do I bear any likeness to my parents? According to descriptions, my father was a harsh and grave man, preoccupied with science; he took little interest in art, and of the poets he would love only Nekrasov and Nikitin. My mother, on the contrary, had a soft, feminine disposition and inherited from her father a love for art and literature, which she passed on to me. From my father, I think, I got the ability to communicate thoughts in talks and lectures, of which I delivered many during a certain period of my life for a wide variety of audiences.

As a 13-year-old child, fascinated by botany and entomology, I lectured to an audience of my nanny, brothers and sisters, and I myself drew tables with a pencil for the demonstrations. Later I made several presentations at the House of Youth in Ryazan in 1916–1918. I consider this ability of mine to make presentations and deliver lectures as an innate talent, perhaps, a hereditary one, which probably roots itself in the constitution of my psyche.
Interestingly enough, my father was not keen on politics and, as far as I know, did not engage in any active work during the revolution of 1905. But he felt the progress of natural science and spoke about it openly and passionately. I cannot make political speeches either, but I could talk endlessly on physics, if only there were listeners. For a long time, I was keenly engaged in lecturing at universities. I even dared to give lecture courses that were challenging for me (in the methodological sense), such as theoretical mechanics, electromagnetic field theory, atomic theory, optics, and even … the history of physics. Interestingly, I would liven up with a small audience of 10–20 people and fumble with a large one, where I could not discern individual people <…>
In the period from 14 to 18 years of age, I had two passions: physics and music, and also literature. All of us boys were passionate about something. I remember that being influenced by my stepfather, I dreamed of becoming a priest. But my persistent passion for physics was unaccompanied by any thoughts of a professional career.
How did it all begin? I remember that by the age of 14 (after the death of my father) I became very apathetic; nothing could interest me. I was sad all the time; I would pity everyone in the evenings. Once he came to class, called me and Shurka Skachkov and ordered us to look after a very large (multi-storey) aquarium that belonged to the gymnasium. So we began to go regularly to the natural sciences study room; we were allowed to take the keys and spend there as much time as we needed. One day I found a door in that room, which was left open solely by accident, and I saw physical instruments, but I did not dare to enter. But one other time, when the door was locked, I climbed up onto Shurka’s shoulders and looked through the window above the door, observing everything I saw with bated breath.

I will skip the story of how I ingratiated myself with the physics teacher L. L. Zakharovsky, who often fiddled around in the physics room. Soon I myself became an integral part of that room; I would stay there after classes and then come back for the whole evening. This passion of mine was soon noticed by everyone, and the director encouraged it dearly.
In first person: creative life path
My creative life was directly and almost inseparably connected with chemical warfare. This is where I gave science everything I was worth.
I came into that field in the spring of 1932, when I was on a quest for where to apply my knowledge. The decisive influence came from my unforgettable impressions from the tracks of alpha particles in a Wilson chamber that I myself designed. Back then no one in Moscow had ever worked with a Wilson chamber, so I decided to create this device at the university in 1928–1929 on my own whim.
My mentor gave me no advice yet allowed me to use the facilities of the Physics Institute of Moscow State University and a radioactive substance (mesotorium). I remember that the very first experiment in the chamber filled me with enthusiasm – the moment when in the light of an arc lamp, clear-cut rectilinear tracks of alpha particles flashed against a dark background. Vigorously working to improve the technique and take photographs, I was drifting ever further away from the topic and immersing myself deeper into the intricacies of cloud tracks.
TATYANA GOLOVENKO:
My grandfather’s youth, which coincided with the revolution and the civil war, was not overshadowed by losses and was interesting and quite peaceful – with a literary circle, with theatrical performances at home, with first scientific experiments…
While still living in Ryazan, he met and soon married Nadezhda Serapionova, a very beautiful girl with a lively disposition and strong character. Raised in a solid healthy family, she was studying at Ryazan Pedagogical Institute. After getting married, the Radushkevichs moved to Moscow, where they had three children.
The family prospered as the grandfather was a military man and served at the Military Academy of Chemical Defense; his career as a scientist and military was on the rise. Right before the war, he was granted an apartment in a new apartment building in the Gospital’ny Val Street. However, those we terrifying times – the Soviet state came, like a tank, after his family too. The first victim was his stepfather, former staff captain M. I. Shcherbakov, who was arrested and shot for befriending a priest. And in 1937 his brother Viktor was exiled to a prisoner camp and soon shot too.
The fate of Leonid Radushkevich himself hung in the balance. His scientific research was classified as he dealt with chemical defenses. Obeying the rules of that life, he hid his noble origins, but he could not part with family heirlooms. In short, there was enough, as they said, to be jailed for.
Every night, they would come and walk someone away in their building, and my grandmother, pregnant with her third child, would lean in fear at the door to look if they had not been coming after them. Like many other people, they kept in a closet a wrapped bundle with necessities, prepared in advance. The fact that my grandfather was spared the tragedy was an utter miracle since even his value as a scientist would not have protected him – there were sharashkas, special prisoner camps for scientists, engineers, and technicians.
We, his grandchildren, remember, most vividly, his sincere interest in all manifestations of life, be it natural phenomena or child-rearing, literature or music. With equal enthusiasm, he would scoop frog eggs from the pond in the spring to show us the development of tadpoles, or catch butterflies, which he identified according to an old catalogue; he would read aloud Chekhov’s stories and put on records with beautiful music. Watching a solar eclipse or a rainbow with childish delight, he would at once explain to us these natural phenomena in a clear and understandable way.
He gave away most of his big professorial salary to his numerous relatives, living a very modest life himself. Careerism was completely alien to him. Many of his coworkers considered him an eccentric, but almost everyone loved him dearly. For his anniversary he was presented with a statuette of Don Quixote…
One does not need to be as wise as Solomon to understand that each track consists of tiniest water droplets, forming an actual aerosol cloud. I leaned that fast and turned to disperse systems in gases. A translation of Gibbs’ Aerosols had just appeared; I purchased the book and set out to read. Surprisingly enough, the new field was familiar to me, since it touched upon gas ionization problems, which I elaborated on during my university years <…>
 My university teaching had become a burden for me by that time; I resented its monotony as I had realized that teaching physics at secondary school was much more exciting than at the university, where a young assistant had no autonomy at all, being entirely dependent on the professor who taught the main course… I tried to find myself a job at a research institute, but I could not see anything that would inspire me. I remember going to the X-ray Institute, intending to work with the emanation machine, but the absence of a clearly formulated science agenda in nuclear physics at that institute drove me off. I was offered a position in infrared spectroscopy research at the Institute of Mineral Raw Materials, but even that place could not provide me with a clear research assignment.
My university teaching had become a burden for me by that time; I resented its monotony as I had realized that teaching physics at secondary school was much more exciting than at the university, where a young assistant had no autonomy at all, being entirely dependent on the professor who taught the main course… I tried to find myself a job at a research institute, but I could not see anything that would inspire me. I remember going to the X-ray Institute, intending to work with the emanation machine, but the absence of a clearly formulated science agenda in nuclear physics at that institute drove me off. I was offered a position in infrared spectroscopy research at the Institute of Mineral Raw Materials, but even that place could not provide me with a clear research assignment.
At about the end of 1931, the Dubinin family were staying at our house. At that time I was head over heels in studying the properties of aerosols arising in the Wilson chamber and spoke about them a lot about with Mikhail Dubinin. Once we went out with him to the kitchen so that he could have a smoke, and he said suddenly that his laboratory had had plans to organize research on aerosols in order to develop defenses against hazardous ones. As a result, in 1932 I got a position as a researcher in the gas defense laboratory of the Chemical Technology Institute (subsequently renamed as the Military Academy of Chemical Defense).
When I came to chemical warfare research, our country was preparing for war, and the Red Army was overhauling its warfare. In those years, chemical warfare was going through a period of stagnation, which was natural, since interest towards it waned after the end of World War I, especially in our country, which prioritized construction and industry development.
Leonid Radushkevich: “I already wrote about my heuristic mindset, which reveals itself in my scientific works. But there is an important correction to be made. When applied to myself, it speaks of clearly manifested amateurism rather than heuristics, which is quite understandable. My studies took place in most troubled times, when there could not possibly be any real system of education. My senior years at the gymnasium coincided with the time when gymnasium education was not only staggering, it was openly ridiculed, and at the university I found myself in a motley mixture of students and old professors of mathematics, who were already leaving. My many years of working as a university teacher would not allow me to go forward; it was only after parting with that work as a 32-year-old man, I could engage in science, on my own. Hence my obvious amateurism – there was no system. However, I have no regrets. Perhaps, under relentless influence of a “venerable elder,” I would not have developed independence thinking”
Chemical warfare research entered a stage of empirical calculations, primitive assessments of impacts of toxic agents, etc. Two years passed since the death of N. A. Shilov, who left behind one single published work on sorption dynamics (1929). His research was handed over to pure chemists, utterly alien to hydrodynamics and mathematical methods. So the works on sorption dynamics continued along a purely empirical path of establishing experimental dependences of Shilov’s equation parameters on the various factors. This trend would long overshadow the physical nature of this equation and the very essence of sorption. Creative thought in this field would lie dormant until 1938.
Even worse was the situation with aerosol filtering. The mechanism underlying this process was entirely unknown although fibrous filters apparently possessed a distinctive property of selectivity. I will never forget the day when, as a gesture of ultimate trust, I was invited to the first laboratory that studied filters. The first thing I saw was a giant collection of filtering materials. Its richness and variety was incredible! It contained most diverse fibrous and granular materials of plant and animal origin and all sorts of industrial waste. Cotton, wool, flax, cellulose, and viscose would lie there next to sawdust, halalite and ebonite shavings, sulfur powders, rosin, fleece from textile factories, etc. (even goose down was treated back then as a highly classified material).

Leonid Radushkevich: “Phraseology and meaning! Lecturing was a sport for me. Perhaps, I could have become an outstanding sworn attorney or a preacher... I would willingly lecture on any topic. All this is a sign of a spiritual ability, rather than an interest in meaning. Another one like that was, I think, Rudin. But one needs such people; they can spark interest in certain things. It is no coincidence that I allured so many people into physics. And that was my main purpose”
One’s understanding of aerosols was vague, blurry, and misguided. There were human trials using tobacco smoke with primitive opacimeters and diphenylchloroarsyl. There were studies of smokes using colossal concentrations in large chambers and smoke bombs. Neighboring fields of chemical warfare showed no success either. Thus, absolutely nothing was done to study the spread of toxic agents in the atmosphere. The design of new such substances followed the well beaten chemical track of synthesizing complex organic molecules, which always yielded substances perfectly absorbed by active carbon.
The chemical defense technology followed, as always, the general level of technology in the country. Back then the chemical defense industry was weak, and so were the means of individual and collective protection. The entire field of chemical warfare would appear so boring to an outsider that it attracted neither the attention nor the effort on the part of scientists. Especially alien would it seem to a physicist. It was a heyday of wave mechanics; the discovery of the positron and neutron spurred colossal progress in nuclear physics; discussions went on about the principle of uncertainty. Naturally, physicists, and subsequently physicochemists, would show much more interest in those questions. Chemical warfare research looked like a dead end business no one wanted to deal with.
This is how a young physicist (I was 31 at the time), with substantial experience in teaching but not in scientific work, plunged into that swamp and began to stir it in all possible ways. All that activity of mine took place among a huge number of typical military characters, mostly soldiers and muscleheads. Completely ignorant in the matters of science, they demanded military discipline and loyalty to the Bolsheviks; they incessantly opposed any manifestations of creative spirit. Many of them were honest fighters, but some were rascals in uniform and plain fools.

LEONID RADUSHKEVICH
Our military chemists could not rise to generalizations, but as early as in the 1920s, German experts emphasized that chemical weapons, unlike artillery, have a volumetric effect, i. e., they spread in volume. However, they too could not clearly formulate the nature of this effect. Therefore, the introduction of a differential equation for convective diffusion transfer as a basic law of military chemistry, which I ascribe entirely to myself, was a real discovery.
This law is based on my observations and interests in two areas: physical kinetics and hydrodynamics of turbulent flows. The first impressions took shape in a poetic setting in the summer of 1936 near Voskresensk. Walking along the flowering valley of Istra, I would sit down by the stream and listen to its murmur. The trickles of water in the grass produced an uncertain sound without any musical tone, and yet they seemed incessantly alternating; they seemed to be in order. Meanwhile, the motion of the flowing water was in complete chaos. Yet this disorder created some kind of order.
The next winter, influenced by these thoughts, I attended Kolmogorov’s lecture on almost periodic functions. But they did not satisfy me, and I began to look into the theory of turbulence. The work by Fage and Towpend* on observing water flow near the wall through an ultramicroscope seemed as the most interesting one to me. In my ultramicroscope, smoke particles stopped when the tap was closed, and I could observe their motion in a vertical gap of only 40 µ, i. e., trace their motion in a layer of 20 µ and below. I often saw them make oscillatory (horizontal) motions, shaped as bright lines. The amplitude of the oscillations kept on decreasing, and the particle turned into a “star” when it settled onto the wall. All that delighted and amazed me. I had to take a closer look at the theory of turbulent motion of gases and liquids, but it proved to be poorly developed at that time. Therefore, I moved on to the theory of atmospheric turbulence, where I saw a way to solve the problem through the diffusion mechanism.
The next step I took at the test site in the summer of 1938. I saw it myself, in the numerous field experiments amid the wide expanses of the steppe, how they spread: gases, vapors, fumes, mists. Those were large-scale experiments, with real toxic agents, in a wide variety of meteorological conditions. The latter was especially valuable, since I chanced to eye-witness how clouds spread during inversion, during ascending currents at different times of the day and night <…> The long night-time experiments did not tire me; moreover, I found the unusual situation captivating. Everything seemed almost magical. Pouring toxic agents from an airplane, site contamination, a massive smoke launcher with adamsite sea bombs, experiments on dogs, smoke screens. Everything was so illuminative and exciting…
Filled with these impressions, I returned to Moscow, where I continued to reflect on the phenomena I saw that summer. I took interest in vortex motion in the atmosphere, but soon I realized that everyone in meteorology was writing about giant vortices, which we do not encounter in the surface layer. It was a heyday of dynamic meteorology, which promised to fully reveal the mechanism of cyclones and anticyclones and allow weather prediction. But we were interested in completely different phenomena and a different part of the atmosphere… The stochastic theory of turbulent processes was only coming into being, and it was clear that the diffusion mechanism would explain a lot…
I do remember well the summer of 1939 – in a close group of friends I was playfully putting together my baggage, drop by drop, like a bee that carries honey from a flower to a hive. And in 1940, the whole picture became clear to me, and everything, literally everything fell into place. The composition seemed perfect, without unnecessary details and yet without any discordant notes or flaws
I remember that one officer from the educational department pressured us to explain our methodology for teaching chemical warfare technology. I dared to say that we had to ground it in the methodology of teaching physics or physical chemistry. He menacingly retorted that that was nonsense and insisted on using the infantry tactical training method, which had been issued as an order. These episodes of misunderstanding happened all the time.
 Now, looking back at the past, I see my 22 years of work in chemical warfare in a different light. In fact, from the outside, I must have seemed like a terrible eccentric, dressed in an outfit incongruent with my figure and appearance. My activity was terribly contradictory in nature. I had no military education; my lax countenance and my inclinations were in no way compatible with the demands raised by the military. I was completely ignorant of military affairs. And they drew attention to that. I remember that one of the teachers said, in the presence of all the staff members, that he did not understand why I was working in chemical warfare and added that it must have been utterly uninteresting and alien to me as a physicist.
Now, looking back at the past, I see my 22 years of work in chemical warfare in a different light. In fact, from the outside, I must have seemed like a terrible eccentric, dressed in an outfit incongruent with my figure and appearance. My activity was terribly contradictory in nature. I had no military education; my lax countenance and my inclinations were in no way compatible with the demands raised by the military. I was completely ignorant of military affairs. And they drew attention to that. I remember that one of the teachers said, in the presence of all the staff members, that he did not understand why I was working in chemical warfare and added that it must have been utterly uninteresting and alien to me as a physicist.
Obviously, given the formal impossibility of leaving the army, this field was still attractive to me, and I kept on working. Facing nothing but opposition, I went deeper and deeper, expanding my field ever wider. It took me a lot of creative energy to work out those general principles that I kept on elaborating over the next two decades. This situation revealed the main features of my creative power – a mind not particularly deep yet really broad, an ability to come up with generalizations, and a passion for heuristic inferences.
Now it is difficult to say what I could have been worth in another field. Most of my life is already behind me; I no longer have that quickness of mind and vigor. In any case, I believe that chemical warfare was fertile soil for sowing new ideas. However, toxic agents were not used in World War II, and my creative experience was buried alive. Still, my ideas could have somehow influenced that decision of the commanders of the opposing countries – the USSR and Germany – since my conclusions clearly showed the main shortcomings of chemical weapons. Thus, it is possible that my negative judgments indirectly influenced the withdrawal from using chemical weapons. If this is true, to an extent however small, then I can consider myself rewarded by the fact that in that war, with all its cruelty, there were no victims of chemical weapons <…>
Now, in 1964, after more than thirty years of working in science, it is time to draw conclusions and give a self-critical evaluation of achievements. With a cold mind, I cast a retrospective look into the past and try to fairly evaluate the results of my creative work.
One’s own evaluation of the importance of their work is often erroneous; this is true of many biographies. They say, Conan Doyle attached more value to his novels and had little appreciation for Sherlock Holmes <…>
The works on filtering come first for me, in terms of scope and duration; they show my persistent interest in these problems, which I have retained to this day, and my most important accomplishments date back to the times after leaving the army. Research on sorption dynamics is described in detail in my doctoral dissertation and, very briefly, in my 1947 article in Doklady Akademii Nauk (The Proceedings of the USSR Academy of Sciences). I think these problems are important, but I myself have done more to pose them rather than develop. Finally, I consider all the other works of mine to be of secondary importance and somewhat shallow.
 As we can see, Radushkevich did not mention the discovery of carbon nanotubes among what he thought to be his main achievements. Moreover, he said not a single word about them in his autobiography. Obviously, he saw them as an intriguing by-product of his studies on aerosol filtering materials. One cannot say it was due to a lack of imagination, just the opposite. Radushkevich himself admitted this feature of his mind and disposition: “There is yet another circumstance that deserves to be mentioned, which, perhaps, characterizes me as a scientist but might seem immodest as regards to my own view of myself. No matter how hard you try to hide your ideas, you have to share them with others. This is especially true for heuristic scientists as they abundantly spawn ideas but resist developing them.
As we can see, Radushkevich did not mention the discovery of carbon nanotubes among what he thought to be his main achievements. Moreover, he said not a single word about them in his autobiography. Obviously, he saw them as an intriguing by-product of his studies on aerosol filtering materials. One cannot say it was due to a lack of imagination, just the opposite. Radushkevich himself admitted this feature of his mind and disposition: “There is yet another circumstance that deserves to be mentioned, which, perhaps, characterizes me as a scientist but might seem immodest as regards to my own view of myself. No matter how hard you try to hide your ideas, you have to share them with others. This is especially true for heuristic scientists as they abundantly spawn ideas but resist developing them.
“The mind so vividly perceives a problem that sometimes it delivers, all of a sudden, or after a short reflection, a solution, albeit often incorrect and shallow one. If, nevertheless, it is successful, then no wonder that others easily grasp it and follow on, although the author himself may already have lost interest in it and switched to other things. This has happened to me ever so often. At various meetings, congresses, or workshops, I would suddenly come up with ideas, and I openly shared them with others. As a result, my ideas were often picked up to grow into serious works, but their authors would either not mention me at all or even blatantly criticize. Sometimes I myself would create a vision but would not go deeper with it or leave it for good.
“Let bygones be bygones. I cannot formally prove it now that I was the author of the ideas that were picked up from me and elaborated on. I have no documentary evidence, so I can only hope that the reader will take my word for it. But this is not evidence of priority in science. Therefore, I cite below some cases of borrowing, not intending to claim my priority but simply desiring to refine my portrait as scientist:
– The theory of formation of cosmic dust in connection with the theory of the Solar System origin (1933).
– The theory of particle codeposition from a stream (colmatage theory) (1934).
– Turbulent coagulation of aerosols (1936), etc. <…>”
Below Radushkevich would write the following remarkable words:
“Again the same damn question arises: Is it possible to reconcile your own evaluation of your work with other people’s opinions? What I believe to be my most important work, others might find uninteresting and insignificant. ‘By their deeds ye shall judge them.’ Ultimately, it does not matter how I arrived at those results. Here the result does not depend on the path, just like the function of state in thermodynamics. This explains the huge difference in how the author himself and others evaluate their work, especially as time goes by. An example is not far to seek – look at the life’s work of Einstein, who could not reconcile the field theory with the theory of matter… I might be wrong but I myself consider the general principles of chemical warfare and the theory of chemical defenses as my main accomplishments.”
Leonid Radushkevich died on November 8, 1972 in the hospital of the Academy of Sciences from a stroke that hit him when he leaned over to the bedside table to get the text of a dissertation for review. He is buried at the Vostryakovskoye cemetery in Moscow, where a gray granite monument stands, which he, with his love of order, had long designed himself, leaving space for his name
The discovery of carbon nanotubes did not become a crowning moment for Radushkevich and Lukyanovich. There was no continuation of that work, and its results, published in Russian, for many years remained unnoticed by the world scientific community. Nevertheless, today we pay tribute to these researchers, who, despite the level of their microscopic technology, were the first to discern in ordinary soot and describe structures that are called today the material of the future. With a bit of luck, their names could have appeared on the Nobel Olympus.
* Translated by Alec Vagapov
** Translator’s Note. The spelling of these names (Fage and Towpend) is an educated guess of the translator as no information was found on this work
References
Kats E. A. Uglerodnye nanotrubki – fantastika nayavu (Carbon nanotubes: science fiction in reality) // Energiya: ekonomika, tekhnologiya, ekologiya. 2008. N. 3. P. 42–49 [in Russian].
Petrov A. K. O nauchnoi etike, Nobelevskikh rezul’tatakh i patriotizme (On scientific ethics, Nobel results, and patriotism) // Nauka v Sibiri. January 17. 2013. N. 2(2887) [in Russian].
Golovenko T. Yu. Kontsert dlya klarneta s orkestrom (Concerto for clarinet and orchestra) // Dedushka, Grand-père, Grandfather... Memories of Grandsons and Granddaughters about Their Grandfathers, Famous and Not so Famous, with Vintage Photographs of the 19th and 20th Centuries / Compiled by E. Lavrentieva. Moscow: Eterna, 2011. P. 48–51 [in Russian].
The editors are grateful to Dr. A. K. Petrov (ICKC SB RAS, Novosibirsk) for the idea of the publication and T. Yu. Golovenko (Moscow) for the photographs and diaries of her grandfather L. V. Radushkevich. This article saw the light through a great effort of Dr. Z. K. Silagadze (INP SB RAS, Novosibirsk), who searched for published and archival materials and prepared a review on the history of research on carbon nanotubes













