Biosilica - Raw Material of the New Millennium
In 1844 the German scholar and traveler A. Humboldt was the first to enlighten the West on Siberia’s richness in natural resources, primarily in precious metals. In our times, this vast land is associated not only with gold and platinum, but also with diamonds, gas, and oil… And yet Siberia’s most valuable treasure is not exhaustible resources of metals, but its unique plants and animals
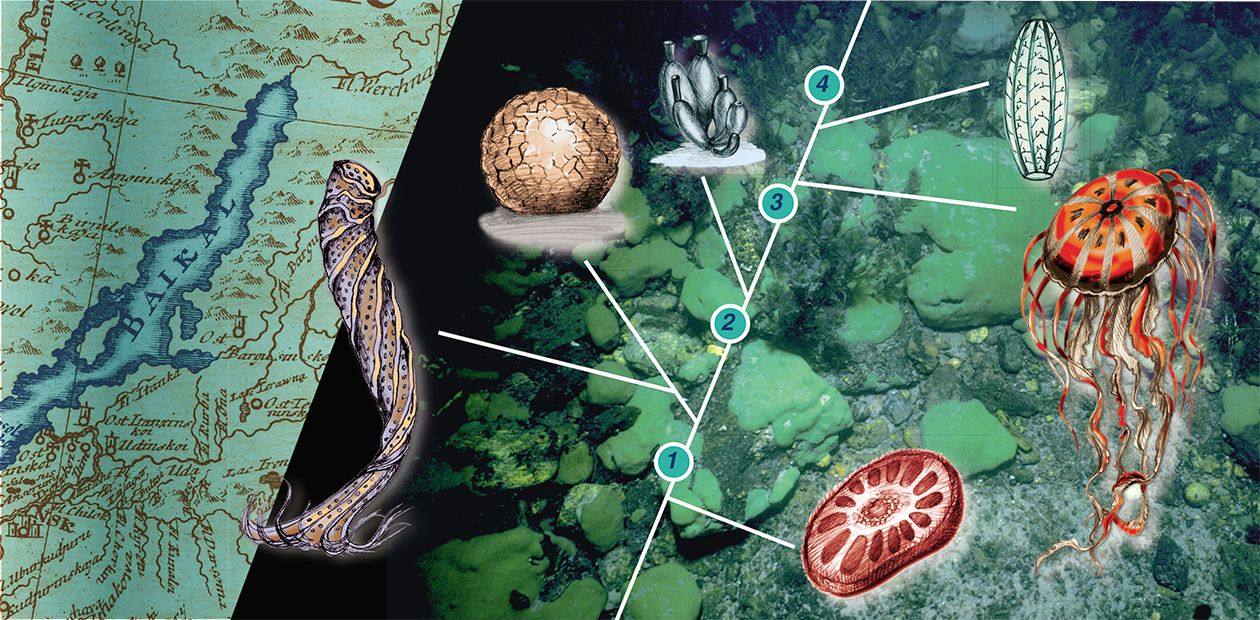
This living treasure is hidden in Lake Baikal, the world’s most ancient and deepest lake and Siberia’s pride. Among its most unusual inhabitants are freshwater sponges. Their first descriptions (Pallas, 1771; Georgi, 1775; Middendorf, 1867; Dybowski, 1880) made it clear that these animals represent a real treasure of nature and disclose basic enigmas of natural science.
These sponges, referred to as “bodiaga” in Russian, have been traditionally used in medicine to heal bruises and in cosmetology to prepare peeling masks resulting in a “natural” blush. Very recent is the discovery of sponges as an inexhaustible source of the precious raw material biosilica, which has an enormous commercial potential, as it can be used to produce silicone materials.
Lake Baikal abounds in this amazing living material of the future. The cooperative research conducted in Irkutsk by the Limnology Institute, SB RAS, headed by Academician Mikhail Grachev and in Germany by the Center of Excellence BIOTECmarin coordinated by Prof. W. E. G. Mller is intended to promote biosilica research to the highest world level
Evolution of Baikal sponges
In 1771 P. Pallas, a scientist and traveler, discovered the first sponge of Lake Baikal and described it as a mucous animal with soft tissue, more than 1.5 ft in size. He termed it Spongia baicalensis and considered it to be related to the European freshwater sponge.
A detailed investigation of Baikal sponges was carried out in 1880 by the exiled Pole W. Dybowsky, who introduced a detailed classification with a separate taxon Lubomirskia. Later it was found that two families of sponges inhabit Lake Bailkal: the Spongillidae, widely spread in water basins, and the Lubomirskiidae. Like most other inhabitants of the lake, all the thirteen species of the Lubomirskiidae, belonging to four genera, are endemic: you will not find them anywhere else (Rezvoj, 1936; Efremova, 2001)
The origin of Baikal sponges has long been an open question. Some scientists believed that the sponges had originated from different marine sponge taxa (Dybowski, 1882; Annandale, 1913; Rezvoj, 1936; Martinson, 1940), whilst others argued that they had a common ancestor and diversified later, in Lake Bailkal (Efremova, 1994). To answer this question on the basis of conventional morphological, embryological, and histological data was impossible.
Help has come from molecular-phylogenetic analysis. A comparison of the nucleotide composition of certain genes of the sponges belonging to the Lubomirskiidae family with that of the cosmopolitan freshwater Spongillidae and of the marine Suberitidae families showed that the Baikal sponges are monophyletic, that is, they share the ancestor (Schrder et al., 2003). It was revealed that even though, on average, the evolution of species takes a long time, it can sometimes (in certain ecological conditions) go at a much quicker pace, and this is what happened at Baikal.
The best model sponge for applied and evolutionary studies which help to unravel Porifera’s genetic growth and origin is the chlorophyll-bearing silicon Lubomirskia baicalensis, the first sponge discovered in Lake Baikal. This widespread sponge dwells on rocky substrates and dominates zones of the lake exposed to light; skin and scuba divers can reach it easily.
The shape of this graceful and well-proportioned animal varies from finger-like to laminated crust. At a depth of 2 m, green velvet cushions of this sponge cover the rocks; at a depth of 5 m its dichotomous branches rise from the encrusted base, and at a depth of 10 m and more you can see Lubomirskiidae sponge “woodland” with some specimens about 1 m high — an unforgettable surrealistic landscape!
Spiky skeleton
Similarly to many other multicellular organisms, the sponge body is supported by a skeleton. As early as the beginning of the 19th century, it was discovered that skeletons of some sponge taxa contain silicon, whilst others are made of calx, which has provided a basis for sponge classification (Nardo, 1833).
In the class of ordinary sponges Demospongiae, to which the Baikal sponges belong, the skeletal elements termed spicules are formed from silica, a silicic acid polymer. Silicaon is the most abundant element of the earth’s crust, widely represented in microorganisms, unicellular and multicellular organisms (plants and animals).
In recent years silicon metabolism occurring in sponge spicules has been elucidated to a large extent (Cha et al. 1999; Krasko et al. 2000; Muller et al. 2003). The formation of the silicon skeleton turned out to be a highly complex process. The formation of spicules occurs in specialized cells, the sclerocytes, where silica is deposited around the organic filament.
The synthesis of spicules is a rapid process: it takes 40 hours to form a 100 µm long spicule embryo (microsclere). The formation of spicules is controlled genetically. Spicule embryos reach practically their full length inside maternal cells; and after they leave them their construction is completed by other specialized cells.
Spicules, those of sea sponges in particular, vary greatly in shape and in size. The spicules of freshwater sponges look like pencils with both ends sharpened. The skeleton of the Baikal sponge L. baicalensis is made of spiky megascleres of various sizes, whilst young spicules are normally smooth.
The skeletal framework of sponges is highly ordered. L. baicalensis grows in a radiate manner, which means that highly ordered growth zones alternate with rest zones.
We have found that at appropriate concentrations in cells silicic acid induces genes encoding various proteins, including enzymatically active silicatein (Krasko et al. 2002). It mediates the apposition of amorphous silica and, hence, the formation of spicules.
Silicatein is located in the axial canal of spicules and can be isolated in pure form by stepwise treatment of the sponges with hydrofluoric acid. A major step to the elucidation of the formation of sponges’ skeletons on the molecular level (and, running ahead, to the creation of silica biotechnology) was the finding that it is this enzyme that makes the core of spicules.
Interestingly, silicatein is located not only in the central axial canal of spicules, but also at the periphery, around the outer concentric silica layers. In the process of spicule formation, silicatein synthesizes around itself a silica shell, up to 2—3 µm in diameter. The new spicules are then extruded from the cells and grow outside in opposite directions through the mediation of silicatein layered onto the surface of the spicules.
The fully developed spicules may be over 10 µm in diameter. This growth process is very similar to the process of bone formation in vertebrates.
Silicatein from… colon bacillus
Spicules may form not only in entire sponge bodies, but also in the so-called primmorphs, a certain form of sponge cell culture. Silicon-responsive genes, including the gene encoding silicatein, are activated if the primmorphs are grown at 60 µM concentrations of a silicon-containing compound.
The axial filament, as mentioned, is composed of silicatein. If the axial filaments, i. e. silicatein, are incubated together with monomeric silicates, the monomers polymerize and form silica depositions on the filaments. Observing this enzymatically controlled process is incredibly fascinating, as it displays the power of biochemical reactions. In the absence of an enzymic catalyst, silica polymerization normally requires high temperatures (1000°C); whereas the enzymatically mediated reaction occurs at room temperature.
And where can we get enough silicatein to meet the needs of biotechnologists? Even though the Baikalian sponges grow relatively rapidly, at least 1 cm a year, a sustainable use of silicatein becomes possible only if gene engineering technologies are involved: the appropriate gene should be transferred to a foreign organism, e. g. a bacterium. And this has been achieved.
We identified and cloned the gene of silicatein of the Baikalian sponge L. baicalensis in an ordinary colon bacillus, and it worked successfully in the new “alien” environment (Kaluzhnaya et al. 2005). The recombinant protein obtained mediated — like the native molecule in the axial canals of sponge spicules — the enzymic reaction, catalyzing the deposition of biosilica.
We obtained a patent for the gene-engineering technology of silicatein generation (Mller et al. 2004). Furthermore, in the course of our study of the metabolism of siliceous spicules, one more protein “reverse” to silicatein, namely, silicase, was identified; silicase is able to depolymerize (dissolve) amorphous silica, which is accompanied by the formation of free silicic acid.
We also managed to generate recombinant silicase and prove its fermentative ability to dissolve silica (Schrder et al. 2003). No doubt, this enzyme holds a great biotechnological potential.
Silicatein applications
Interestingly, local population has always used the Baikalian siliceous sponges to polish silver and copper kitchenware (Pallas, 1787) and as a cleaning detergent. Today, biosilica applications range from the coating of artificial bones to the creation of various tissue implants.
The need for biosilica is huge. The data on the ability of sponge spicules to synthesize biosilica enzymatically (using the enzyme silicatein) and to degrade it enzymatically (using the enzyme silicase) indicates that possible applications in biotechnology have not yet been completely appreciated. It is already certain that applications of these biocatalysts will range from the production of silicon-based materials in optics and electronics to biomedicine, biocatalysis, and other fields.
The focus will be on personal care products, fabric care, cleaning products and — in a longer run — on the application as biosensors, in protein engineering and biocatalysis. Silicatein will be used to form new biomaterials and functional medical compounds, which can be applied, among other things, in the controlled drug delivery systems. It is regarded as a most promising material of the third millennium.
Achievements in silica biotechnology have been made owing to a combination of the Mainz expertise in molecular biology, Irkutsk highly trained specialists in limnology, and Lake Baikal itself, as a unique biotope, whose dissolved silica has preserved and promoted the evolution of sponges, one of the most ancient and amazing water creatures. The impact of this triarchy in silica biotechnology cannot be quantified — it is enormous.
It is Baikal, the Siberian freshwater “sea”, that has provided the basis for the Russian-German collaboration. In fact, this cooperation in its many forms, including academic, dates back to the 18th century when the German well-known naturalist and traveler participating in the Second Kamchatka Expedition I. Gmelin noted that there was no town in Russia where people would not come to exchange their goods (1751).
Times have changed, but German scientists — like Gmelin — continue to come to the far-away Siberia, though not to explore this unknown land but to work, in cooperation with their Russian colleagues, in the highly advanced biotechnological fields.
References
Itskovich V. B., Belikov S. I., Efremova S. M., Masuda Y. Phylogenetic relationships between Lubomirskiidae, Spongillidae and some marine sponges according to partial sequences of 18S rDNA// Memoire of the Queensland Museum 1999,44: 275—280.
Schrder H. C., Efremova S. M., Itskovich V. B., Belikov S. I., Masuda Y., Krasko A., Mller I. M., Mller W. E. G. Molecular phylogeny of freshwater sponges in Lake Baikal// J. Zool Syst Evol Research, 2003, 41: 80—86.
Kaluzhnaya O. V, Belikov S. I., Schrder H. C.,
Wiens M., Giovine M., Krasko A.,
Mller I. M., Mller W. E. G. Dynamics of Skeleton Formation in the Lake Baikal Sponge Lubomirskia baicalensis. Part II. Molecular Biological Studies//Naturwissenschaften 2005, 92 (3): 134—138.