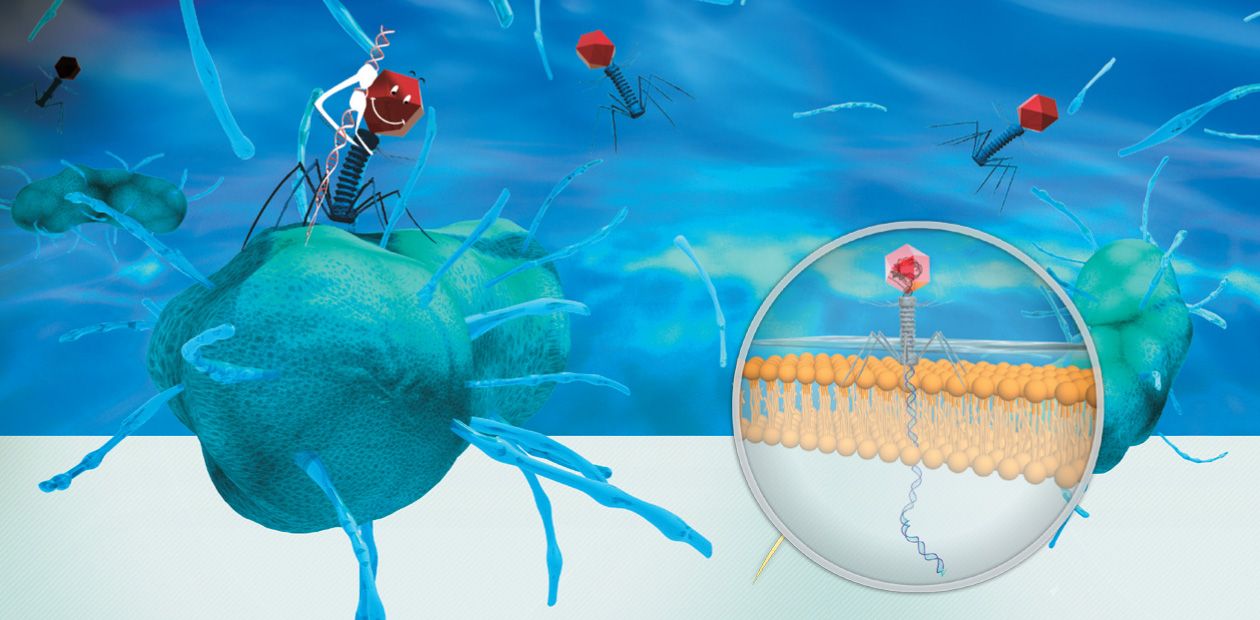
Bacteriophages: The Enemies of Our Enemies
Bacteriophages (in Greek, those that devour bacteria) or just phages are the viruses able to kill bacteria. According to the latest data, the main role played in nature by these “bacteria killers,” which are the most abundant cell organisms on Earth, is to considerably accelerate organic matter decomposition (eventually, to carbon dioxide and water). Thus, by influencing the global geochemical processes, phages maintain the matter and energy exchange in the Earth’s biosphere. As for humans, since many bacteria are our enemies, it looks reasonable to use phages as an efficient and safe “biological weapon” for eradication or control of hazardous and pathogenic bacteria
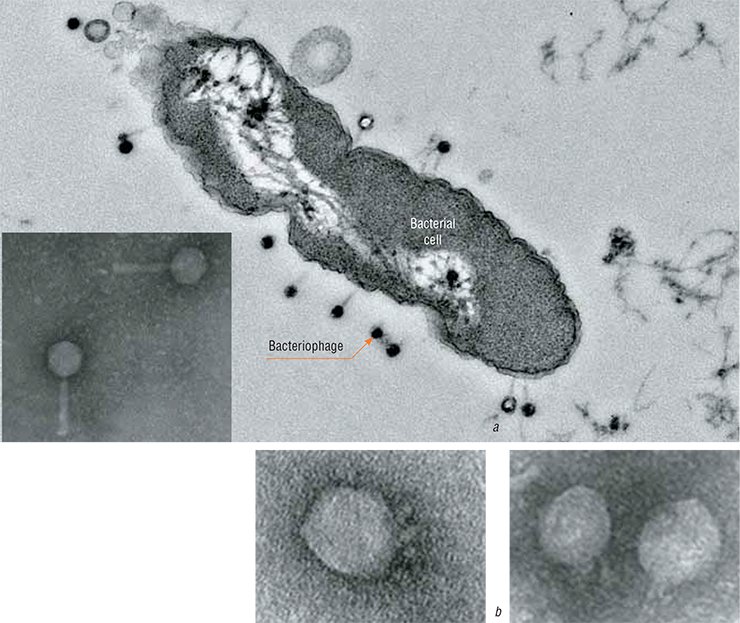
Similar to all viruses, bacteriophages border on the living and the inanimate. Outside the host cells, most bacteriophages exist as virions, objects resembling intricate molecular crystals; they “live their lives,” i. e., they reproduce only in bacterial cells, since the bacteriophage genome is insufficient for autonomous existence.
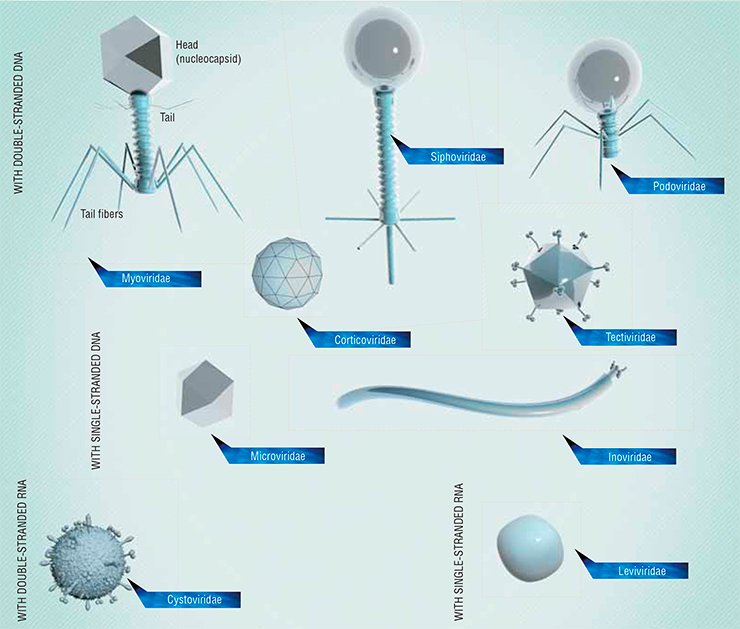
The world of bacteriophages is diverse: over 500 bacteriophage species of manifold shapes and structures are known today. In relation to bacteria, phages may be either temperate or lytic. Temperate phages insert their genomes into those of bacteria and do not immediately manifest their “murderous” activity: hiding in the bacterial genomes, they reproduce together with their hosts and then kill the hosts’ numerous progeny. As for lytic phages, they kill bacteria immediately after infecting them. The latter simple phages are currently most attractive objects for biotechnological and therapeutic purposes.
Some more statistics: assuming that an average diameter of a phage particle is 50 nm, all bacteriophages of the globe put in line will cover a distance of 5 × 1020 kilometers or 5 × 107 light years! This is the distance to Virgo Supercluster, nearest to us, while only 4.2 light years separate us from the nearest star, the Proxima Centauri.
Bacteriophages were discovered over one hundred years ago almost simultaneously in different countries. As early as the late 19th century, several researchers (N.F. Gamaleya, a Russian microbiologist, among them) discovered some minute agents able to pass through the pores of porcelain filters and kill bacteria.
In 1915, F. Twort, an Englishman, and F. d’Hérelle, a Frenchman, succeeded in isolating these agents (afterwards, d’Hérelle spent many years struggling with Twort in attempts to prove his priority). It was d’Hérelle who named the discovered particles “bacteriophages.” He immediately grasped their potential as antibacterials and pioneered in administering a phage preparation for treating dysentery in children as early as 1919
Combat “vehicles”
Bacteriophages, these natural combat vehicles, have a most simple structure: their genetic material, be it DNA or RNA, is packed in a protein envelope equipped with “weapons,” special tools for attacking bacteria.
The genome of different phages may vary in length. Some phages have very short genomes, as small as 3.5 kilobase pairs, comprising information about only three to four proteins. Moreover, the genes encoding these proteins have to overlap. The genome sizes of other phages may be comparable to that of large, intricately organized viruses of multicellular animals, reaching 170 kilobase pairs in length (the largest known phage has a genome of 480 kilobase pairs). Such a genome can code for up to 200 different proteins and such phages are large and complex in their structure.
 Since olden times people have encountered cases of miraculous healing of infectious diseases. Today, we have grounds to explain some of such instances by occasional use of bacteriophages. Note that these bactericidal agents are ubiquitous over the globe, being present in soil and water, and muds and mixtures of natural products have been used for healing inflammations and wounds since ancient times. Similar cases of miraculous healing may have given rise to such ceremonies as bathing in the holy waters of the Ganges or baptizing in the Jordan River.
Since olden times people have encountered cases of miraculous healing of infectious diseases. Today, we have grounds to explain some of such instances by occasional use of bacteriophages. Note that these bactericidal agents are ubiquitous over the globe, being present in soil and water, and muds and mixtures of natural products have been used for healing inflammations and wounds since ancient times. Similar cases of miraculous healing may have given rise to such ceremonies as bathing in the holy waters of the Ganges or baptizing in the Jordan River.
Also, the antibacterial activity of natural products was used not so long ago. In the 1920s, Holy Archbishop Luka (Voino-Yasenetsky), a talented surgeon, when being in exile in a remote village, got to know about the local sorceress who could heal septic wounds and carbuncles with a mixture of soil, sour milk, and ash. Luke tried to understand the nature of this method by experimenting with the composition of the remedy. However, the treatment results were poorly reproducible (as well as the results of all researchers who worked with bacteriophages at that time). Sometimes this mixture led to an amazingly rapid recovery, but in some cases it did not help at all. Lacking suitable facilities for his work or the possibility to read medical literature, Luka had to cease his research. Nowadays we know that the efficiency of a phage preparation will depend on whether it contains the bacteriophages specific for the pathogenic bacteria of an individual patient
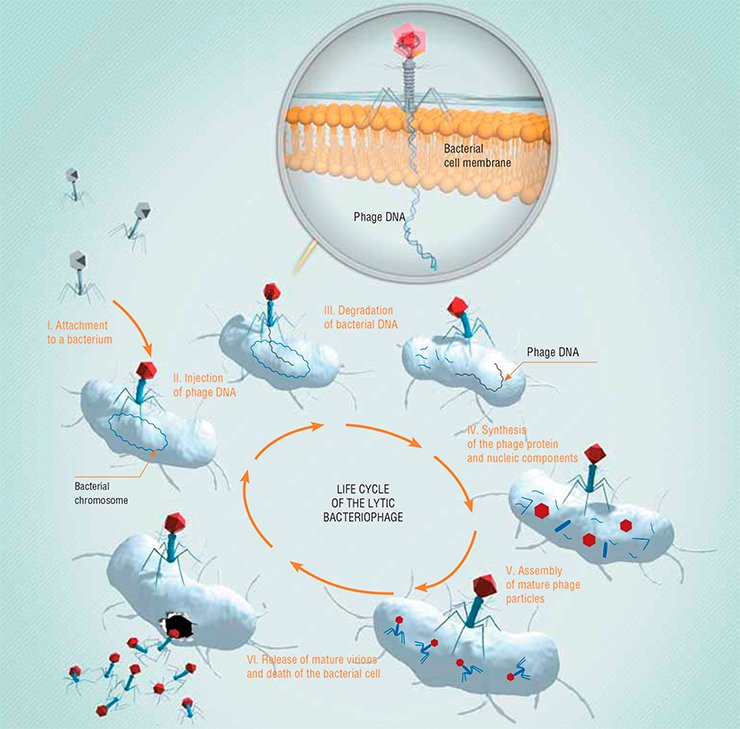
A lytic phage in its appearance is most similar to a robot spacecraft able to dock with a target object. The phage head, a protein container with packed DNA, is connected with the “tail”, which is a protein structure resembling tentacles and housing the so-called recognition elements, capable of binding with receptors (specific proteins or polysaccharides) on the surface of bacteria.
After docking with the target bacterium, the phage sticks to its surface. Hundreds of phages may simultaneously attack one bacterium; however, a single phage is sufficient to kill it. Note that the phage–cell interaction is very specific. A particular phage can interact only with its target bacterial species and is unable to attach itself to human cells.
Once a phage settles itself on the bacterial surface, its tail punctures the bacterial wall with the help of specialized proteins. Through the tail channel, the genetic material is injected into the cell. The phage genetic programs take control over the cell’s life-supporting systems, switching all host material and energy resources, as well as molecular “machinery” to synthesizing phage proteins and genome copies.
The bacterium will die. In the next half-hour, hundreds and thousands of new phage particles are synthesized; then the cell is destroyed, releasing another brigade of bacterial killers.
Arms race
According to estimates, bacteriophages kill half of the world’s bacterial population every two days. That is why bacteria, known for their rapid reproduction, have not yet covered the entire surface of our planet with a thick layer: if there were no phages, it would take them a few days.
Despite this highest antibacterial efficiency of phages, bacteria have not vanished from the face of the earth. The fact is that these two types of organisms have coevolved over many millions of years; this coevolution, though, might be called an “arms race.” Thanks to mutations, phages constantly “invented” new warfare tools and tricks, while bacteria, in turn, “invented” ingenious protection means.
For example, the DNA of all bacteria carries special labels (methylated bases). Besides, specialized bacterial enzymes, restriction endonucleases, cleave any DNA lacking such labels, including phage DNA. However, some phages learned to simulate such labels, thus escaping the bacterial defense.
Another protective mechanism used by bacteria involves changes in surface receptors: in this case, a phage just cannot recognize “its own” bacterium. In response, there appear phages whose recognition elements have been altered so that they are capable of binding with new bacterial receptors. Phages and bacteria have several mechanisms for such modifications of their genetic programs. Mutations may appear randomly during genetic replication. In addition, genetic recombination allows different phage species to exchange fragments of their genomes and even grab fragments of bacterial genomes.
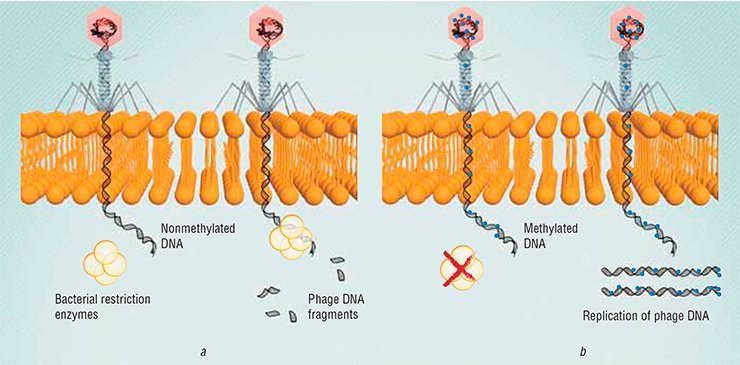
In addition, changes in the receptors of a pathogenic bacterium, from a medical standpoint, can be fortunate for humans, since bacterial receptors are the virulence factors that determine survival and reproduction of a pathogen in the host body. Moreover, bacterial mutants resistant to phages are usually less pathogenic than their progenitors.
Phage therapy: per aspera
Discovery of viruses that kill bacteria gave rise to a new method for the control of bacteria. The most evident application is phage therapy, i.e. the use of bacteriophages for treating human bacterial infections. An advantage of this approach is a most stringent specificity of phages, affecting only their “favorite” infectious agents.
For medical purposes, bacteriophages were first used as early as 1915, when Félix d’Hérelle, one of their discoverers, used such a preparation to cure dysentery in children. However, the further history of phage therapy was anything but easy. The approach suggested by d’Hérelle was too much ahead of his time, so he had to spend many years struggling for the recognition of his discovery. This struggle involved his famous French colleagues, who denied d’Hérelle’s views on the nature of these bactericidal agents, regarding them as enzymes. The truth triumphed only in the 1940s, but d’Hérelle, tired of the struggle, had left for the United States long before.
In 1934, d’Hérelle came to Tbilisi (Georgia), where they had put in operationunique facilities for developing phage therapy. From 1918, there had been a laboratory of microbiology there led by George Eliava which later became an institute, Eliava had been sent to the famous Pasteur Institute to master new techniques and to purchase equipment; there he met d’Hérelle and got acquainted with his amazing discovery.
Thus, Eliava started to dream about founding a world center for study of bacteriophages in Tbilisi. Josef Stalin took interest in this project, and a building for the future Institute of Bacteriophages, Microbiology, and Virology (now named after Eliava) was constructed and equipped in 1930. However, the further events followed a typical USSR scenario of those times: in 1937 Eliava and his wife were arrested; he was shot as an “enemy of the people” while d’Hérelle returned to Paris. Nonetheless, the institute survived and successfully continued to work.
In the early 1940s, they started to use phage therapy in Europe and in the United States. Millions of patients received such preparations; however, the treatment results were ambiguous and irreproducible. The advertised miracles failed, and the very idea of phage therapy was discredited. The reason was that not only the manufacturers, but also the scientists themselves lacked the necessary knowledge about phage properties and about the mechanisms of their function, to say nothing of the reliable technologies for manipulations with viruses.
With failure after failure, pharmaceutists and physicians could not but sigh with relief with the advent of antibiotics. These relatively inexpensive chemical substances with a wide range of antibacterial effects and a long shelf-life seemed to radically solve the problem of infectious diseases. In western countries bacteriophages were forgotten for many years. The French company for manufacturing commercial phage preparations founded by d’Hérelle switched to other projects (in particular, the famous cosmetics and beauty company L’Oreal originated from it).
The phage research was continued only in the Soviet Union, Poland and Czechoslovakia. The largest manufacturer of phage preparations was the Georgian institute founded by Eliava: by the 1980s, about 1200 people worked there and the preparations were sent for clinical trials throughout the Soviet Union. Bacteriophage production was also organized in Ufa and Gorky (now Nizhni Novgorod).
In addition, a political aspect also played an important part in cessation of phage research abroad. Given that phage therapy developed in the Soviet Union, it was politically “incorrect” for western scientists to be involved in the research associated with the name of Stalin. This was the time of Lysenkoism, when western science had a skeptical attitude towards anything done by Soviet biologists.
Phage cocktails
Recent years brought about a new round of interest in phage therapy. Antibiotics have not become a panacea when dealing with bacterial infections: at present, the design of new drugs fails to keep up with the increasing number of bacteria that acquired resistance to the existing antibiotics. As of today, 40 % of staphylococcal infections in the United Kingdom hospitals are caused by such strains, and about 90 000 patients die annually from hospital infections caused by drug-resistant bacteria in the United States. If we recalculate these rates per the world population, it will be three to five million deaths per year.
The World Health Organization alerts the world of the coming “post-antibiotic” era, when there will be no drugs to treat common bacterial infections. Against this background, phage therapy looks the most promising direction that could lead to effective personalized treatment methods. The necessary knowledge about phages and about the mechanisms of their interaction with bacterial cells as well as the technologies for working with viruses is now available.
Currently, only virulent lytic phages are used in phage therapy, namely “tailed” bacteriophages of the order Caudovirales and filamentous phages belonging to the families Leviviridae (with single-stranded RNA genome) and Inoviridae (with single-stranded circular DNA genome).
As is mentioned above, the host range of phages is usually very narrow, being confined to one or several closely related bacterial species. On the one hand, such a strict specificity is good for therapy, since it allows for elimination of a particular organism without interfering with the overall bacterial community of the human body. On the other hand, in the case of an emergency (when there is no time to identify the particular bacterium that has caused an infection in a wound or on a burned surface), it is necessary to have medicine that would simultaneously affect several bacterial species that are potentiall pathogens. Phage cocktails – a mixture of several phages differing in their host specificities – are usually applied.
This approach was used by d’Hérelle. The d’Hérelle cocktail, which he brought from Paris as early as 1930, is still one of the main phage preparations, being the major component of the Georgian Pyophage and Russian Intestiphage. In Tbilisi, they used phage cocktails to design preparations for treating gastrointestinal diseases and septic wounds. The drugs were intended for use during epidemics or military actions. Army trials and a large-scale experiment conducted in Tbilisi on preventing gastrointestinal distress in children, demonstrated good efficiency of such cocktails.
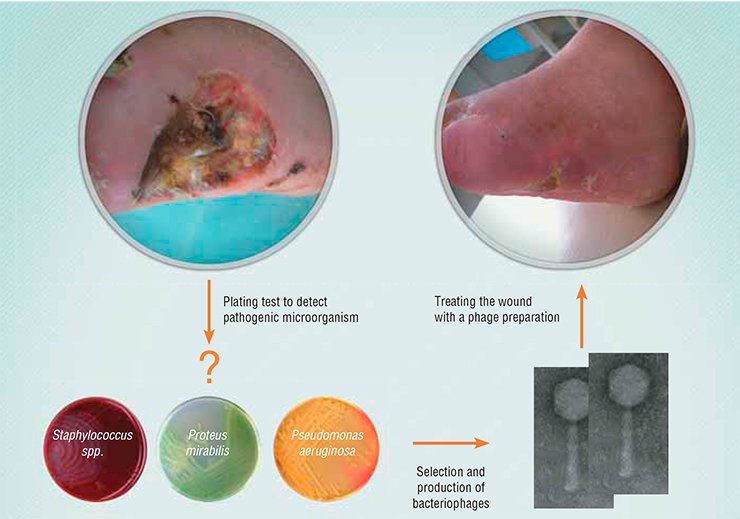
Phage cocktails are produced as standard preparations targeted at the bacterial communities frequently occurring in commonly known diseases. Indeed, more effective cocktails will result if the components are selected to hit the bacterial community of an individual patient . In order to compose such a custom-made cocktail, it is necessary to test the patient’s bacteria for sensitivity to the phages from the collection in order to select the needed strains. If the required phages are absent, they should be searched for in natural substrates.
In general, the search for bacteriophages is rather simple. Specimens from various sources–aquatic bodies, soil, sewage runoff – are applied to a bacterial culture. If the bacteria are killed, they are removed by centrifugation, and the remaining solution is tested for its activity. Then the phage is propagated by cultivation in the corresponding bacterial culture. Moreover, phages may be vacuum-dried and administered in capsules. In such a form, the phage preparation retains stability for 14 months at a temperature of up to 55 °C.
Modern history
So far, the broadest experience in phage therapy has been acquired by the specialists from Tbilisi and a specialized center, the Ludwik Hirszfeld Institute of Immunology and Experimental Therapy (Wroclaw, Poland); there, they produce small batches of bacteriophages intended for trials.
From the very first Polish researchers have focused on personalized therapy. They have used phages for experimental treatment of chronic cases after unsuccessful antibiotic therapy. This center has already had thousands of patients, many of whom have been completely cured.
The clinical trials have demonstrated a high efficiency of phages in treating infectious lung diseases: a single intranasal portion of a phage preparation is sufficient to inhibit a throat, nasal, or lung infection. Phages are no less efficient in eliminating pathogenic bacteria from the gastrointestinal tract. A high efficiency of phages has been also shown for almost all cases of pyogenic ulcer of diabetic foot, lung diseases, mastitis, and urogenital infections. The list of diseases may be continued; moreover, no side effects caused by bacteriophages have been observed.
In the United Kingdom, phage preparations have been successfully tested for treating chronic otitis, a malady hard to cure, owing to the so-called microbial films of drug-resistant bacteria. Research in this field is almost absent in France, the cradle of phage therapy, although the Pasteur Institute did, until recently, produce custom-built phage cocktails.
The Russian company Mikrogen manufactures phage preparations on a large scale. They are available in the drugstores of Russia, Belarus, and Ukraine. Phage preparations manufactured by Mikrogen and the Tbilisi Center for Burn Infections successfully passed trials in Belgium.
Nonetheless, bacteriophage therapy has not been yet officially approved in most countries: neither the FDA (US Food and Drug Administration) nor analogous European agencies have given their approval. Within the European Union area, phages are used for treating patients only at the above mentioned Institute of Immunology and Experimental Therapy (Poland).
Thus, the interested patients are treated in a medical tourism mode. The Phage International Inc. (California, United States) directs patients from various countries, those who suffer from chronic diseases caused by drug-resistant bacteria, to either the Tbilisi Center for Phage Therapy or to its own clinic in Mexico.
Pick out phages
Why isn’t phage therapy in common use yet? It is evident that antibiotics should be the drug of choice for acute life-threatening cases when there is no time to select a specific agent, whereas bacteriophages as more friendly agents are preferable for treating chronic infections.
Skepticism of many experts, especially of those from western countries, is one of the major factors hindering the medical use of phages. Following the unfavorable tradition, western scientists still distrust the positive research results of the Soviet Union times, although they themselves have fallen behind in this particular area.
However, a stronger reason is that large pharmaceutical companies are not interested in bacteriophages. These companies want exclusive rights to inventions, whereas phages are natural agents hard to patent; besides, the very idea of phage therapy was patented long ago. In addition, these companies have made huge investments in the production of antibiotics, so they do not want any competition with inexpensive phage-based drugs.
For example, in 2006 the FDA approved bacteriophage cocktails for treating meat and other agricultural foodstuff. In this case, phages were regarded as food supplements. Phages were also approved for use as disinfection agents.
Phage aerosols have been successfully tested in experiments on protecting poultry on large farms as well as fish, in fish farms
As for physicians, they have been routinely taught to prescribe wide-range antibiotics in order to attain the maximum effect. In contrast, phage therapy implies a large toolkit of drugs to be tested and individually selected for each patient. Eventually, this considerably increases the cost of such a personalized treatment. Moreover, though people continue to die of infections caused by drug-resistant bacteria, this market is rather small from the economic standpoint and treatment of such diseases is fraught with legal implications.
On the other hand, the FDA now admits that phages, owing to their high specificity and nontoxicity, may help where alternative approaches fail. Correspondingly, the FDA plans to work out practical recommendations for using lytic phages in therapy. For this purpose, it is necessary to sequence their genomes, outline safe cultivation conditions, and perform up-to-date standard animal tests for their toxicity. If a phage cocktail is to be used, each of its components should be characterized. Finally, to prove the efficiency of such preparations it is necessary to conduct controlled clinical trials.
Thus, phages may find a considerably wider use than we currently imagine. In particular, state-of-the-art techniques are able to construct phages that produce antibacterial toxins and directly deliver them into bacterial cells. Modern genetic engineering allows of a real phage design, for example, constructing phages that display an altered or broadened specificity. Moreover, synthesizing a complete phage genome is now quite feasible.
Individual phage components and substances used by phages to kill bacteria are now undergoing tests as antibacterial drugs. An example is bacteriocins, fragments of phage tail structures, which damage the bacterial cell wall forming pores, so that the cell rapidly loses vitally important ions and dies.
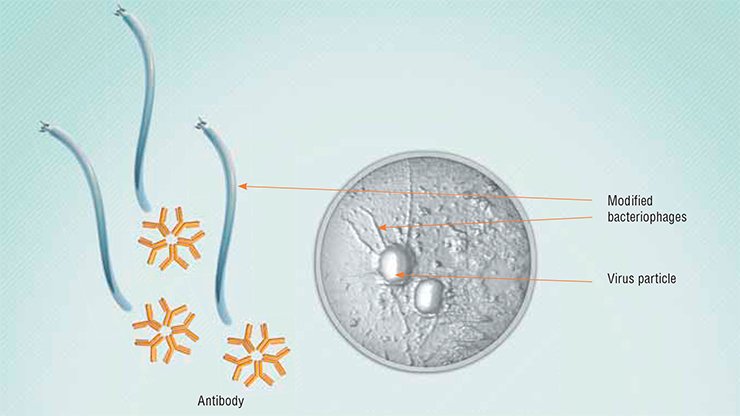
Another developing direction is the use of phages for drug delivery, be they antibodies or chemical therapeutics. It is possible to load a phage particle with up to a thousand molecules of antibodies or several thousand molecules of antibiotics. Moreover, attaching polyethylene glycol molecules to the surface of a phage allows such a phage to circulate in the blood for a long time. In addition, the phages carrying certain peptide antigens on their surface may be used as vaccines.
ANTIBIOTICS ADVANTAGES:have a wide range of antibacterial activity and are easily patentable
SHORTCOMINGS:
affect a patient’s own microflora, which brings risk of secondary infections;
are unable to focus on the infected site;
cause side effects, including allergies and peptic disorders;
induce emergence of drug-resistant bacterial strains;
require much time and money for the design of new antibiotics BACTERIOPHAGES ADVANTAGES:
highly specific (it is possible to find a killer bacteriophage for any bacterium);
search for a new phage takes just several days or weeks;
phage production is inexpensive and ecologically friendly;
do not cause dysbiosis;
are nontoxic and cause no side effects;
are eliminated from the body after killing the pathogen
SHORTCOMINGS:
have a too narrow selectivity: to guarantee successful treatment it is necessary to identify the pathogen;
are hard to patent owing to the diversity of agents
It is evident that stimulating phage therapy advances in the 21st century should be realized at state level. It is necessary to create all the required conditions for constructing and maintaining a repository for the phages that act against antibiotic-resistant pathogens. As for all types of approval documentation, the problem has quite a simple solution: phage preparations may be regarded as similar to influenza vaccines. Indeed, live influenza vaccines, which are cocktails of several virus strains with a constantly changed composition, are produced every year. Official approvals are granted to these virus cocktails; so, why not use this scheme for phages?
The current situation is Russia is unique: phage therapy is officially permitted, and a wide range of “individual” phage preparations and phage cocktails are produced. Why are they so rarely used? The main reason is the personalized approach mentioned above. Indeed, standard phage preparations do not guarantee positive results; and once a preparation fails, this not only disappoints the physician in charge, but also may put the patient at risk in the case of an acute disease.
The way out is evident: it is necessary to test the bacteria of an individual patient for their susceptibility to a phage preparation. This requires well-equipped centers with phage collections and facilities for microbiological testing. Provided the development of a network of such centers under the program for personalized medicine is realized, our country stands a good chance to be among the leaders in this most important field of biomedicine.
References
Kozlova Yu. N., Repin V. E., Anishchenko i dr. Shtamm bakteriofaga Pseudomonas aeruginosa, ispol’zuemyi v kachestve osnovy dlya prigotovleniya asepticheskogo sredstva protiv sinegnoinoi palochki // Patent RU 2455355 C1. 2011.
Tikunova N. V., Morozova V. V. Fagovyi displei na osnove nitchatykh bakteriofagov: primenenie dlya otbora rekombinantnykh antitel // Acte Naturae. 2009. № 3b. S. 6—15.
Kropinski A., Lingohr E., Moyles D., et al. Endemic bacteriophages: a cautionary tale for evaluation of bacteriophage therapy and other interventions for infection control in animals // Virology J. 2012. V. 9. P. 207–215.
Miedzybrodzki R., Borysowski J., Weber-Dabrowska B., et al. Clinical aspects of phage therapy // Adv. Virus Res. 2012. V. 83. P. 73–121.
Summers W. Bacteriophage therapy // Annu. Rev. Microbiol. 2001. V. 55. P. 437–451.
The work was supported by the Center for New Medical Technologies (Akademgorodok, Novosibirsk, Russia)