Personalized Medicine: Treating the Patient, Not the Disease
The well-known principle of treating the patient and not the disease was set forth as early as by Hippocrates, the famous Greek physician. However, many of us have had a bitter experience showing that the current medicine focuses on treating “individual symptoms” of a certain average Joe. Nonetheless, there is light at the end of the tunnel: in our postgenomic era, state-ofthe- art achievements in molecular biology have given rise to the concept of personalized medicine, which is becoming ever more widespread. This gives us hope that a personalized approach to medical treatment will at last become a reality
Even the ancient healers with their vague knowledge about the functions and structure of the human body considered it important to treat their patients differentially. Trying to restore the “natural harmony” or the “balance of body fluids” of a patient, although not always successfully, they did their best to take into account the patient’s body type, life style, nutrition pattern, and even specific features of the dwelling.
Later, with the development of the so-called classical medicine and advent of several new natural science disciplines, such as anatomy, physiology, biochemistry, and pharmacology, physicians learned to cope with numerous maladies earlier regarded as incurable. However, this has made medicine in many respects “impersonal,” and a real suffering individual was replaced by a “typical” patient. Only in some cases (for example, blood transfusion or tissue transplantation) taking into account individual specific features of the patient was a must.
Nonetheless, any development, as we know, follows a spiral pattern. Therefore, it is no wonder that the term individualized (personalized) medicine has become ever more frequent in specialized literature starting from the end of the last century. This refers to a new model in the organization of medical aid that allows for the selection of diagnostic, therapeutic, and prevention methods optimal for a particular individual, which takes into account his/her specific genetic, physiological, and biochemical features. The final aim of such a medicine is to make disease prevention and treatment most efficient, safe and cost-effective.
Molecular biology as a fulcrum
The outstanding advance in molecular biology during the last decades has formed the background for the development of personalized medicine.
The starting point was the famous international Human Genome Project, commenced in 1990. The goal of this project was to determine the complete nucleotide sequence of our genome and to precisely localize individual genes there. The results obtained under this project, which, in particular, brought about an explosive development of massive DNA sequencing techniques, have allowed medical genetics to come close to clinical practice.
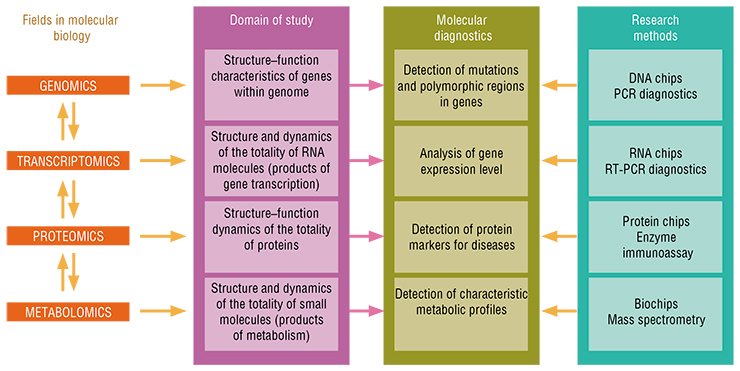
The further development of personalized medicine is tightly associated with emergence of new biological disciplines that deal with different levels at which the information encoded in the genome is implemented, namely, the transcriptome, representing the totality of gene transcription products; proteome, the set of all protein molecules; and, finally, metabolome, the set of relatively small metabolic molecules, final products of metabolism.
The pharmaceutical applications of the above listed disciplines—pharmacogenetics, pharmacogenomics, and pharmacoproteomics—have become the most important bases for personalized medicine. The difference between pharmacogenetics and pharmacogenomics is that the former studies the variation of patients according to their response to drugs and, correspondingly, its goal is to select the adequate therapeutics for the patients with certain genetic profiles. As for the latter, this discipline deals with the development of new drugs based on the studies into specific impacts of various compounds on gene functions at the level of the whole genome.
Why are these disciplines so important? The matter is that currently, when medications are prescribed without taking into account the individual specific features manifesting in response to drugs, 20 to 75% of the patients develop inadequate response to pharmacotherapy or undesirable consequences of drug action. According to the American Medical Association, such adverse side reactions in the United States in 2004 caused hospitalization of 2 million people and over 100 000 lethal outcomes (Zyryanov et al., 2008).
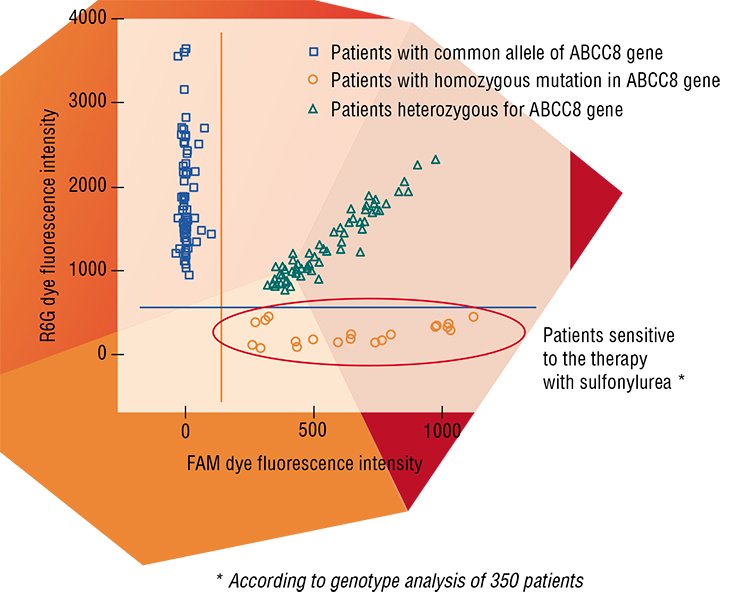
The reason underlying such phenomena is that the drugs in individual persons are subject to metabolism in different ways. For example, the rate of drug elimination from the body may differ several tenfold! However, being aware of the specific features of genotype and the processes of genetic information “deployment” at an organismal level, it is possible to design the prognostic tests allowing the therapeutic efficiency to be assessed before the therapy is started as well as the probability of adverse reactions of individual patients to be estimated.
Selection of an optimal pharmacotherapy at the earliest stage of treatment helps a patient to better comply with the treatment regimen and, which is no less important, reduces medical expenses. Admission of the fact that the individual response to a pharmaceutical actually exits is the first step to therapy personalization and optimization.
“Hi-tech” diagnosis
The most important element of personalized medicine is molecular diagnostics. This in no way rejects the classical diagnosing methods, such as history taking, examination of patients, and clinical study, but focuses on a hi-tech analysis of biological macromolecules (DNA, RNA, and proteins).
Several aspects of molecular diagnosis may be considered in the perspective of personalized medicine, namely, early disease detection, selection of adequate therapy with a safe and efficient drug, integration of diagnosing and therapy, therapy monitoring, and prognosis.
Molecular diagnostics is widely used for genetic testing as well as in genetic screening of large populations. In particular, the newborns are now massively screened in maternity hospitals to detect congenital diseases. A classical example of such a disease is phenylketonuria; in this country, the rate of this disease is one child per 8000—10,000. The children with phenylketonuria may develop mental retardation; however, a timely diagnosis allows for the prevention of this pathology by merely excluding the products containing the amino acid phenylalanine from the child’s diet.
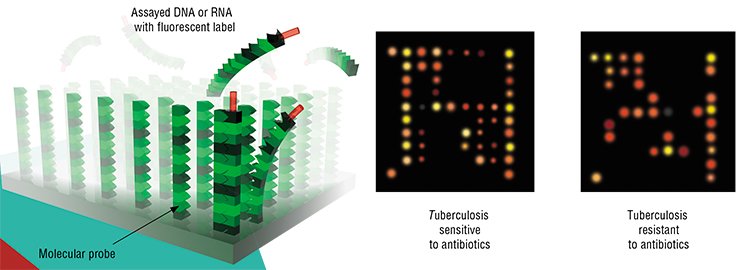
The methods of molecular diagnostics utilize the so-called biomarkers, that is, certain biological molecules found with the help of special studies that are able to indicate a particular state of the target body systems or tissues. The gene regions responsible for certain character of the organism, for example, specifying predisposition to a disease, are also used as biomarkers. In this sense, the most important technologies in the perspective of personalized medicine are genotyping of gene polymorphism (variation) in single nucleotides and biological microarrays (microchips) allowing for detection of extended DNA and RNA nucleotide fragments in clinical samples.
Biochips are miniature devices intended for concurrent recording of manifold interactions between various molecules. The background for this technology is the complementarity of molecules, that is, the ability to bind according to a key–lock principle. Numerous so-called molecular probes are in a strict order immobilized on the plate of a biochip; these probes selectively react with certain regions in target molecules in a clinical sample of a patient.
The biochip microarray technology has immeasurably expanded the diagnostic potential of personalized medicine, making it possible to design portable devices for molecular diagnostics; in addition, a single chip simultaneously houses manifold probes targeted to different molecules. This technology also allows for a considerable acceleration in the development of new “personalized” drugs.
Cancer therapy
Oncology is now the leader in the adoption of various personalized medicine elements. It is now possible to determine a number of cancer markers in a tumor during a surgical intervention or biopsy to design the treatment strategy for an individual patient. The specific mutations of certain genes that determine the severity of disease and its prognosis have already been discovered for breast cancer, melanoma, and some types of lung and colon cancers.
An illustrative example of such biomarkers is the receptor protein for the so-called epidermal growth factor 2 (HER2). An increase synthesis of the HER2 protein is observed in every fifth case of breast cancer and 15% of gastric cancer; in this case, the disease develops in an aggressive manner. This specific feature of a patient is now detectable with the help of a special test, allowing a specific blocking agent to be prescribed (Yamauchi et al., 2001).
Another example here is a mutation in the BRAF gene, which is involved in the regulation of cell division. This mutation occurs in approximately half patients suffering from metastatic melanoma. A special drug has been developed for this case too, which decreases the disease severity (Flaherty, 2010).
 Interferon is used in the treatment of some cancer types; however, such a therapy is successful only in 20—30% of the cases, causing severe adverse reactions in the remaining patients. Molecular diagnostics with the help of a Hoffmann-LaRoche biochip makes it possible to detect such patients. These patients can be treated with interferon at the early disease stages, whereas interferon administration was earlier considered acceptable only in the extreme, neglected cases (Jain, 2002).
Interferon is used in the treatment of some cancer types; however, such a therapy is successful only in 20—30% of the cases, causing severe adverse reactions in the remaining patients. Molecular diagnostics with the help of a Hoffmann-LaRoche biochip makes it possible to detect such patients. These patients can be treated with interferon at the early disease stages, whereas interferon administration was earlier considered acceptable only in the extreme, neglected cases (Jain, 2002).
One of the approaches to treating malignant tumors consists is making the immune system of the body recognize and destroy the cells of a formed tumor. Here, it should be taken into account that although all the tumors emerge according to a similar molecular genetic mechanism, they are usually accompanied by random mutations in different genes. As a result, the “molecular fingerprint” of a tumor, to which the immune system responds, is unique for each individual patient. Antigenics Inc. utilized this fact to start the production of “absolutely personalized” drugs for cancer immunotherapy based on the tumor tissues of individual patients (Jain, 2002).
It is known that the response of individual patients to chemotherapy also considerably varies, presumably being determined by genetic differences in metabolism. The corresponding testing of patients could help to avoid painful and inefficient treatment.
Unfortunately, Russia, especially its remote regions, currently does not have enough clinical laboratories able to test tumor samples from every patient. In this context, the advance in pharmacogenetics in our country is impractical, although it is expected that molecular diagnostic technologies taking into account the specific features of all used anticancer drugs will emerge within a decade.
For the heart and vessels
Another scourge of our time is hypertension, which belongs to the so-called multifactorial diseases. Manifold pharmaceuticals have been developed for its treatment, including diuretics, α-adrenergic blocking agents, calcium channel blocking agents, and others. They differ in their modes of action, efficiencies, and the side effects they cause. Selection of effective and sufficiently safe set of drugs for an individual patient is frequently a complex and nontrivial task for a physician. Thus, it is not surprising that a good control of blood pressure is attainable in less than one-third of all hypertension patients (Rusnak et al., 2001).
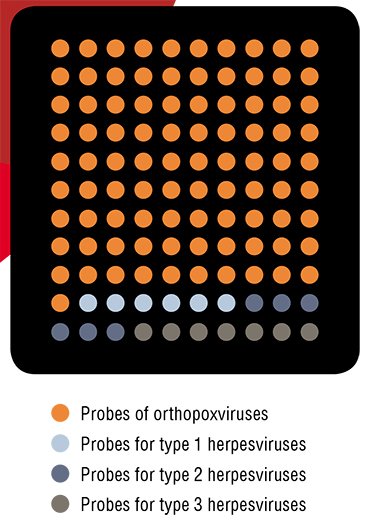
MINIATURE DIAGNOSTICIANThis microchip is able to identify not only the orthopoxviruses pathogenic for humans, but also herpesviruses of types 1–3. The importance of such simultaneous diagnostics is in that the diseases caused by these viruses have similar clinical manifestations but require different therapies
To solve this problem, the possibilities provided by personalized medicine have recently become involved. In particular, it has been found that polymorphism of the gene encoding angiotensin-converting enzyme (ACE) influences the efficiency of its inhibitor, the drug named fosinopril (Stavroulakis and Makris, 2000). Sequenom now manufacturers a test for detecting the patients who should be first of all treated with the drugs displaying an ACE antagonistic activity. Such a testing makes it possible to reduce the number of drugs necessary for an adequate therapy.
It is commonly known that an elevated level of “bad” cholesterol in blood is among the major factors in the development of angina pectoris, a form of ischemic heart disease. The statins (simvastatin, atorvastatin, etc.) are used to decrease this level. In 2002, Genaissance completed a large-scale study aimed at the search for specific genetic markers associated with the efficiency of these drugs. They succeeded in discovering about a hundred of genes potentially associated with the patient’s “response” to statins (Jain, 2002).
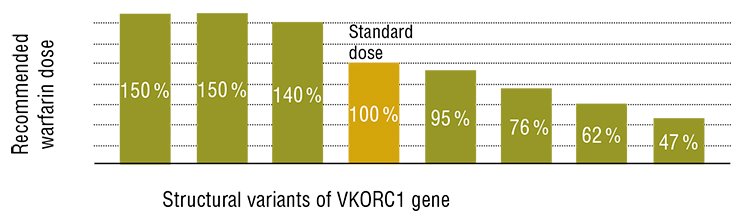
There are many other examples that demonstrate the application of advanced genomic technologies to the individualization of medical treatment. It is assumed that personalized medicine in the nearest future will be actively developed in the medical fields such as cardiology, immunology, and diseases of the central nervous system. In a long-term perspective, the potential of personalized medicine will be used in treating metabolic disorders and even respiratory diseases.
Teething problems
Specific technological problems existing in molecular diagnostics are tackled constantly. However, we still underestimate how complex it is to transform the tremendous volume of research data into novel clinical products.
As sad as it may sound, the majority of biomarkers discovered during the last decade cannot be yet utilized to improve the available clinical tests. The matter is that any potentially significant biomarker should be reproducible, sensitive, and specific. Therefore, it should be initially validated using hundreds of clinical tissue samples, necessarily implying the availability of large collections comprising clinical samples of patients and the corresponding databases. The technological solutions designed for detecting the corresponding marker require similarly long and expensive validation.
Thus, it is no wonder that most of the discovered biomarkers are only mentioned in research papers. For example, a simple search for “cancer marker clinical validation” in the Medline database gives only 1592 entries versus almost 30 000 entries for “new cancer marker.”
It is mostly this painful point that slows down the advance in modern personalized medicine in Russia, and it is in this particular area that massive efforts must be made to overcome the existing problems.
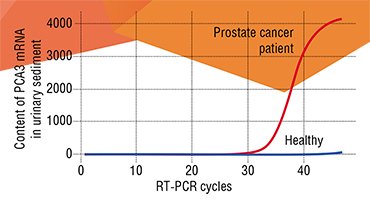 There are also other, more objective, barriers, in particular, incompleteness of the currently available basic knowledge about the correlation between the genotype and phenotype, that is, the actual implementation of hereditary information at the level of an individual. The present technical level in bioinformatics does not always allow for an adequate processing of tremendous data massifs.
There are also other, more objective, barriers, in particular, incompleteness of the currently available basic knowledge about the correlation between the genotype and phenotype, that is, the actual implementation of hereditary information at the level of an individual. The present technical level in bioinformatics does not always allow for an adequate processing of tremendous data massifs.
The economic prospects of the novel medical technologies adopted into medical practice is still rather vague. Marketing experts experience difficulties with assessing the cost for such treatments; however, there are reasons to believe that the mass use of personalized treatment technologies should pay back with time owing to an increased diagnostic efficiency and, eventually, a decrease in the time span required for treatment and rehabilitation.
So what can physicians and their patients expect from personalized medicine?
New portable diagnostic devices will appear on the market. Due to further advance in the informational sphere, patients will get access to their medical data in an electronic format, the genetic profile included.
The possibility to determine genetic predisposition to certain diseases will allow physicians to conceive a set of individual preventive measures and once a disease has commenced, to prescribe the treatment matching the genetic constitution of an individual patient, including the drug tailored for this patient.
Indeed, this is an ideal situation. As for Russia, the development of personalized medicine in this country is first and foremost associated with the activation of pharmacogenetics research into socially significant human diseases, such as cancer and cardiovascular disorders, the chemotherapy for which is especially complex and expensive. The experts believe it necessary to initiate the creation of electronic expert systems and banks of biomaterials as well as implementation of long-term epidemiological projects, which are now completely absent in Russia (Baranov, 2011).
In addition, it is most important to carry out large-scale educative activities for medical and paramedical staff as well as pharmacists and develop new academic programs for higher education institutions focusing on the possibilities and technologies of personalized treatment. Finally, patients themselves as the “final consumers” of medical services should have the possibility to get reliable information about the limitations and advantages of this new medicine.
References
Fedorova O. S., Koval’ V. V. Protemika – vysokotehnologichnaja rybalka // NAUKA iz pervyh ruk. 2010. №2 (32). S. 84—90.
Koptjug A. V., Mamontov E. V., Suhovej Ju. G. Na puti k personalizirovannoj medicine // NAUKA iz pervyh ruk. 2011. №2 (38). S. 90—97.
Koptjug A. V., Mamontov E. V., Suhovej Ju. G. Na puti k personalizirovannoj medicine. Dinamicheskaja model’ razvitija opuholi // NAUKA iz pervyh ruk. 2011. № 6 (42). S. 44—51.
Lifshic G. I., Novikova Ja. V. Terapija: personal’naja doza // NAUKA iz pervyh ruk, 2010. №2 (32). S. 91—94.
Sinjakov A. N. Diagnoz – delo tehniki! // NAUKA iz pervyh ruk. 2007. №5 (17). S. 40—49.
Chernonosov A. A. Krasnorechivye metabolity // NAUKA iz pervyh ruk. 2010. №2 (32). S. 84—90.
Davies K. The $1,000 Genome: The Revolution in DNA Sequencing and the New Era of Personalized Medicine. New York: Free Press, 2010. 340 p.
Jain K. K. Personalized Medicine. Basel: Jain PharmaBiotech Publications, 2003.
Jain K. K. Textbook of Personalized Medicine. New York: Springer, 2009. 419 p.