Regenerative Medicine: Searching for "Elixir оf Life"
Regeneration (from Latin word “regeneratio” for renewal) is a natural restoration of cells, tissues, and organs. Certainly, unlike, for example, the salamander, a human cannot restore their lost limbs, but the so-called physiological regeneration constantly takes place in our body: cells of almost all tissues constantly renew, broken bones knit, and wounds heal… Not long ago it was believed that restoration of organs injured by serious pathologies, such as chronic renal failure or diabetes mellitus, was unfeasible in principle. However, tremendous upgrowth of cell technologies during the last decades has opened alluring prospects for regenerative medicine in the cases previously regarded as incurable
Regeneration (from Latin word “regeneratio” for renewal) is a natural restoration of cells, tissues, and organs. Certainly, unlike, for example, the salamander, a human cannot restore their lost limbs, but the so-called physiological regeneration constantly takes place in our body: cells of almost all tissues constantly renew, broken bones knit, and wounds heal… Not long ago it was believed that restoration of organs injured by serious pathologies, such as chronic renal failure or diabetes mellitus, was unfeasible in principle. However, tremendous upgrowth of cell technologies during the last decades has opened alluring prospects for regenerative medicine in the cases previously regarded as incurable
The majority of cells in our body have strictly determined, unique functions. For example, myocytes, the cells constituting muscle tissue, synthesize the contractile protein myosin; blood β-lymphocytes produce specific immunoglobulins that control infections; neurons, nervous system cells, are able to generate electric pulses, and so on. All such cells have their own specific morphological features, i.e. they are highly differentiated cells unable to transform into one another.
However, the body contains cells of another kind, stem cells. The stem cell does not belong to any particular tissue but is able to transform into a specialized cell, a neuron, myocyte, hepatocyte, etc. These universal stem cells, capable of dividing infinitely giving rise to cells of any body tissues, have become one of the central players in regenerative medicine, the state-of-the-art field in therapy that focuses on regenerative processes in the body.
The earliest, nonspecialized, stem cells are the fertilized egg cell and the cells it produces in several cycles of its first divisions. These cells are referred to as totipotent (from Greek words totus for “entire” and potentia for “power”).In further body development, these cells acquire more specialized properties, being transformed into multipotent stem cells. Such cells are able to give rise to cells of one of the so-called germ layers, namely, to ectoderm, which later develops, in particular, into skin and nerve cells; mesoderm, the progenitor for connective and muscle tissue cells; or endoderm, developing into visceral organs, such as the liver and lungs.
The stem cells that are progenitors of some “akin” cell types are referred to as oligopotent and of a single cell type, unipotent.
The adult body has hematopoietic stem cells (progenitors of all blood cells) as well as stem cells for skin, skeletal muscles, myocardium, and nervous system. All these stem cells “accommodated” in the corresponding tissues display a high potential for proliferation and differentiation; moreover, this also refers to the cells housed in some other tissues. For example, skin stem cells are able to differentiate into neurons
Cell technologies: pros and contras
Initially, all narrowly specialized cells in the body are formed in the course of embryonic development from unspecialized stem cells, just as branches and leaves grow from the tree trunk. However, stem cells are present not only in the embryo: they ensure the development of a child, and throughout life time are responsible for renewal of tissue both in the case of natural loss of cells (for example, the population of erythrocytes is renewed every three—four months) and injuries.
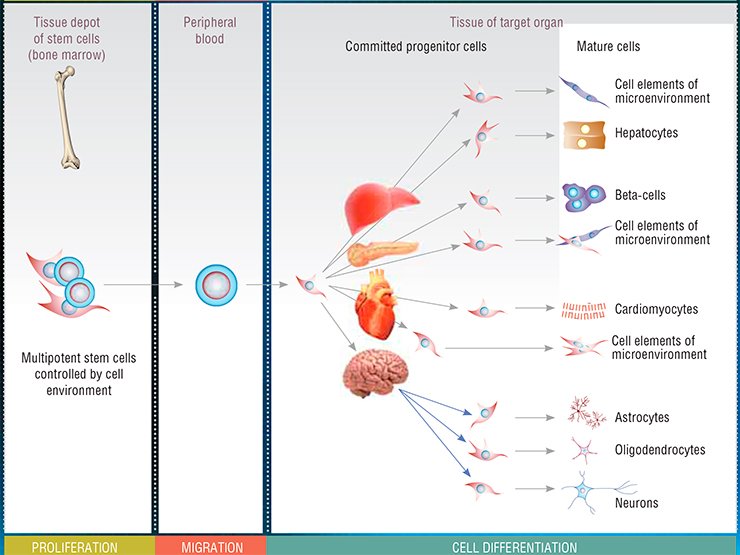
The best studied stem cell population in the adult body is that of bone marrow multipotent mesenchymal stem cells. These cells are able to migrate with blood to remote organs and differentiate into many specialized cell types. Restoring the population of damaged and dead cells in a tissue, they restore the structure and, consequently, function of the corresponding organ.
During last decades, the insight into the properties and activity patterns of stem cells has induced a new approach to treating many diseases, namely, cell therapy. Although this field is currently among the most popular in the world medical science, there are many problems to be solved. The ideal cells for therapeutic purposes are the stem cells of a 4- to 7-day-old embryo, since they do not induce graft rejection. However, this is fraught with a risk of carcinogenesis to say nothing about the ethical issues associated with using embryonic cells.
Thus, the most rational (and best adjusted to human physiology) approach to solving the problems in regenerative medicine is stimulation of the functions of endogenous, i.e., the body’s own stem cells, thus simulating activity of its own natural regulatory systems. The Institute of Pharmacology, Siberian Branch, Russian Academy of Sciences (Tomsk), is involved in this kind of research, namely, in designing pharmacological tools acting on endogenous stem cells.
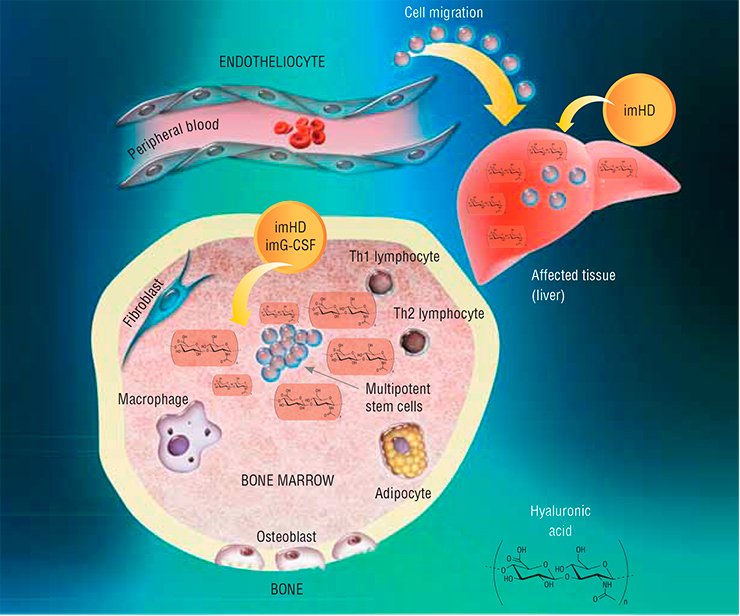
This work started with searching for an answer to the question: how do stem cells of an adult organism respond to severe pathological states? Several pathologies, such as acute hepatitis after the onset of liver cirrhosis or diabetes mellitus, were modeled in experimental animals. The researchers recorded several marker parameters, in particular, the number of multipotent stem cells in the bone marrow, in peripheral blood, and in the involved organ.
It has emerged that regardless of the type of damage, the number of hematopoietic stem cells in the bone marrow increases; however, any increase in their release into blood, migration to the affected organ, or differentiation into corresponding cell types was not observed. Thus, the task was to force endogenous stem cells to do their best; this problem was solved with the help of substances that under natural conditions are also involved in hematopoiesis.
Resource deployment
Granulocyte colony-stimulating factor (G-CSF) is a polypeptide that stimulates proliferation and triggers differentiation of hematopoietic cells belonging to the granulocyte lineage (erythrocytes; segmented leukocytes: neutrophils, basophils, and eosinophils; and cells of the immune system: monocytes and macrophages). In addition, this factor is able to stimulate deployment of bone marrow multipotent mesenchymal stem cells, their migration to target organs, and further cell differentiation.
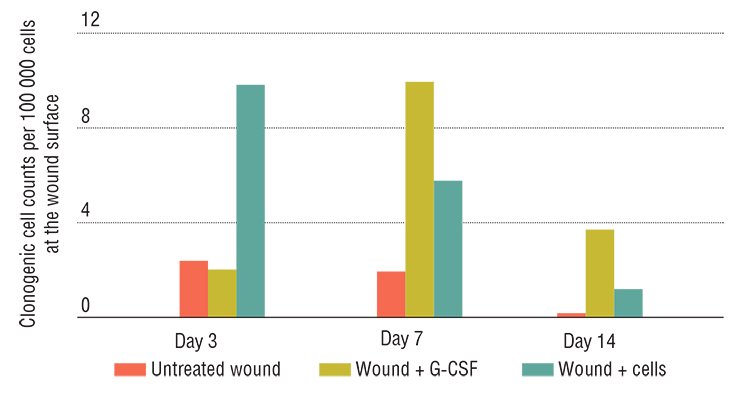
Hyaluronidase, the enzyme that degrades hyaluronic acid, a component of the intercellular matrix (the substance filling the space between cells), has an even wider range of activity. In its native form, hyaluronic acid interacts with specific stem cell receptors; being cleaved into fragments by the enzyme, this acid activates cell division, differentiation, and migration. As a result, hyaluronidase indirectly increases the activity of bone marrow mesenchymal stem cells and hematopoietic progenitor cells as well as boosts G-CSF activity.
The idea to utilize these biologically active substances for activating stem cells to the level sufficient for regenerating particular injured organs seems most attractive. However, both G-CSF and hyaluronidase are very toxic in the doses exceeding their normal content in the body (though necessary for such stimulation); this toxicity rules out their wide use as a tool in regenerative medicine.
Fortunately, the researchers have found a way out: it is necessary to use specialized carriers for these substances. Designing carriers for drugs is a real application of nanotechnologies in pharmaceutical practice. Indeed, here we do not speak about certain futuristic products, such as state-of-the-art nanorobots working inside the human body. A relatively simple manipulation – attachment of pharmacologically active substances to long-lived polymeric molecules—makes it possible to enhance the ability of therapeutic molecules to overcome tissue barriers, to reduce their negative side effects (toxicity and allergenicity), and to increase their efficiency via extending their “lifespan” in the body.
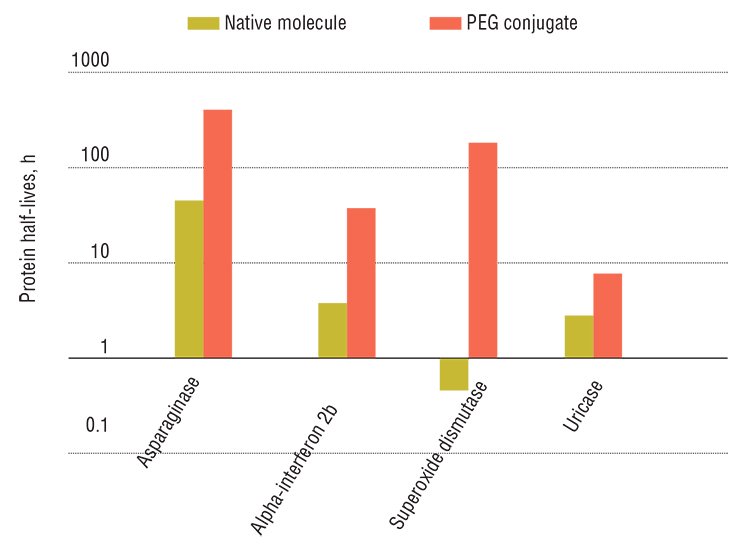
Note that classical technologies used for producing “drug + carrier” complexes are very expensive. However, specialists of several Novosibirsk research and production institutions—Institute of Nuclear Physics, Siberian Branch, Russian Academy of Sciences; Institute of Cytology and Genetics, Siberian Branch, Russian Academy of Sciences; and Scientific Future Management, Ltd. – have elaborated a novel technology for radiation (electron beam) synthesis, allowing for a severalfold decrease in the cost of producing such preparations while retaining high efficiency.
"Pure" ones are losers
Using the technology of radiation synthesis, the Institute of Pharmacology in collaboration with the Scientific Future Management has elaborated nanotechnology-based function modifiers for stem cells. These modifiers involve the same G-CSF and hyaluronidase but immobilized on (chemically bound to) polyethylene glycol (PEG).
Such modified preparations have demonstrated not only higher efficiency as compared with “pure” substances, but also differed from the complexes in the mode of action. In particular, it has emerged that the G-CSF immobilized on polyethylene glycol has a more pronounced effect on the proliferation and differentiation of committed hematopoietic stem cells, which are more specialized as compared with multipotent cells. This reduces the risk of depletion for this cell population, which serves as a “deep reserve” for regeneration in the bone marrow. Although it is so far unclear how dangerous for the body it is to decrease the pool of undifferentiated multipotent stem cells caused by their artificial activation, it is reasonable, in this case, to err on the side of caution.
Organic nanoconstructs are safe: besides preparations within such constructs are more stable, better soluble, and less toxic. They are more efficient as compared with unmodified drugs owing to a longer elimination half-life. However, the PEGylation technique, commonly used for synthesizing such preparations, is a complex, multistage, and expensive process. In addition, this process requires highly toxic reagents, which entails multistage purification of the final product.
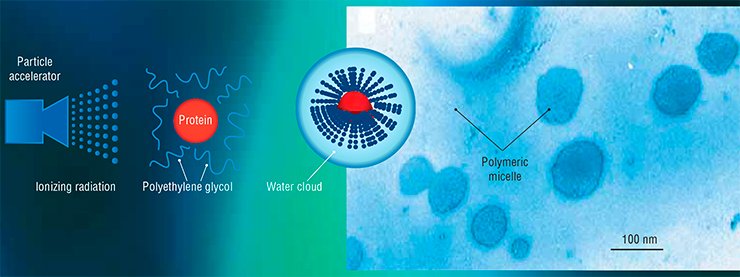
PEGylation is a physiochemical modification of the medicinal molecule of protein nature by linking it to molecules of polyethylene glycol (PEG), a polyatomic alcohol with a linear polymer structure. Owing to high hydrophilicity of PEG, a “water cloud” is formed around the modified preparation, thereby increasing its bioaccessibility and protecting it from the immune system.
Unlike the classical chemical PEGylation, the radiation (electron beam) synthesis “connects” the drug molecule to PEG with the help of an ionizing radiation flux. This may be accelerated electrons, protons, neutrons, gamma-radiation, ultraviolet, laser radiation, or any other type of radiation with a certain spectrum and energy of several megaelectron volts.
Ionizing radiation activates the polymer molecules so that they covalently interact with the nitrogen atoms located in certain positions in protein molecules. Varying the exposure dose, it is possible to produce conjugates of different molecular weights and stereochemical structures, thus altering the properties of the target preparation.
The radiation PEGylation takes only 2—10 min. If the pharmacologically active component is “not afraid” of radiation exposure, the process is completed in a single step. Otherwise, the polymeric carrier alone is exposed to radiation and is then mixed with the active medicinal substance
The preparations of natural hyaluronidase (in safe doses) are effective only when locally administered. As for the modified hyaluronidase, it displays systemic effects on the body as it is resistant to destruction by the corresponding enzymes. The modified hyaluronidase stimulates various kinds of stem cell to proliferate and differentiate and induces them to enter the blood and migrate to target organs in the case of pathologies.
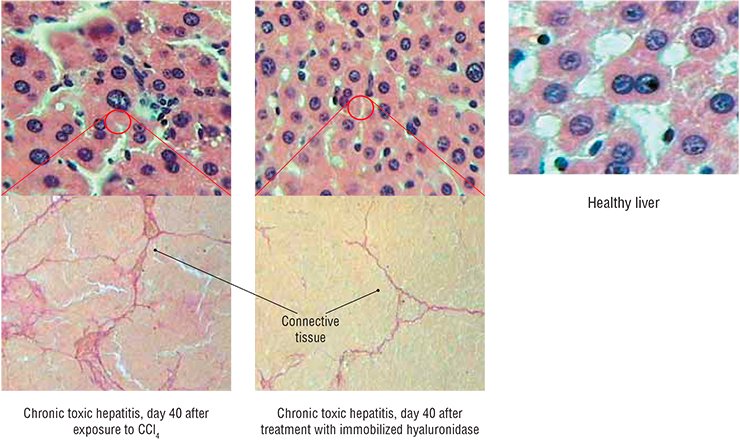
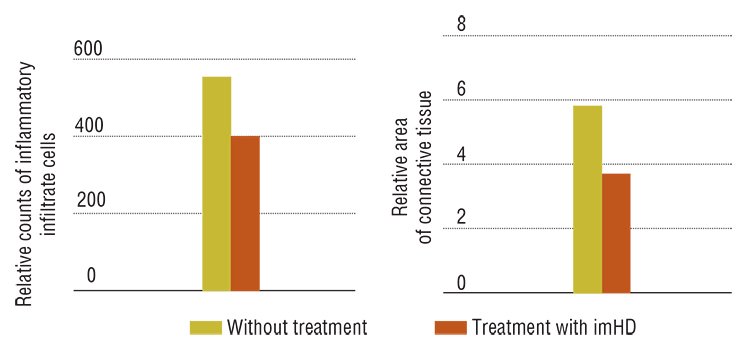
The results obtained with immobilized hyaluronidase are impressive: in the animal model of toxic hepatitis, five-time administration resulted in an almost complete restoration of the liver function and morphology. Note that the necessary doses of this preparation are very low; besides the drug can be taken both orally and by injections.
The advent of cell technologies granted a real hope to the humankind, the hope of healing many maladies so far regarded as incurable. The pharmacological modifiers for stem cell functions elaborated by Siberian researchers confirm that this approach makes it possible to normalize the structure and, correspondingly, the function of almost any organ, provided there is a timely intervention in pathology. For example, experiments have demonstrated a successful treatment of the initial stage of liver cirrhosis. The insulin-free therapy of diabetes mellitus is also being developed: it may happen that endogenous stem cells will allow for restoration of the impaired pancreatic structure to the degree that it will be able to produce sufficient amount of insulin. Of the widespread pathologies involving the nervous system, the most promising for this kind of treatment are encephalopathies, with potential restoration of the brain cognitive functions; and in more remote future, several most severe pathologies, such as Parkinson’s and Alzheimer’s diseases, might be cured.
Certainly, these projects are still at the stage of preclinical research: it will take a long time to create the complete cycle of a new drug; it will also require huge investments. Nonetheless, it is known that a journey of thousand miles begins with a single step…
References
Dygai A. M., Artamonov A. V., Bekarev A. A. i dr. Nanotekhnologii v farmakologii. M.: Izdatel’stvo RAMN, 2011. 136 s.
Dygai A. M., Zyuz’kov G. N. Kletochnaya terapiya: novye podkhody // Nauka v Rossii. Moskva: Nauka, 2009. T. 169, № 1. S. 4—8.
Dygai A. M., Zyuz’kov G. N., Zhdanov V. V. i dr. Immobilizirovannyi granulotsitarnyi koloniestimuliruyushchii faktor. Farmakologicheskie svoistva i perspektivy ispol’zovaniya. Tomsk: OOO «Pechatnaya manufaktura», 2011. 149 s.
Dygai A. M., Zhdanov V. V., Zyuz’kov G. N. i dr. Gepatoprotektornye effekty immobilizirovannykh preparatov granulotsitarnogo kolonie¬stimuliruyushchego faktora i gialuronidazy i mekhanizmy ikh razvitiya // Kletochnye tekhnologii v biologii i meditsine. 2012. № 1. S. 14—18.