The Postgenomic Era. Cryoarchiving by the Example of Mustelids
Along with Homo sapiens, over 4 600 mammalian species are currently existing on the Earth. However, many extant species are in danger as is indicated by the IUCN Red list (htpp://www.redlist.org)
Along with Homo sapiens, over 4 600 mammalian species are currently existing on the Earth. However, many extant species are in danger as is indicated by the IUCN Red list (htpp://www.redlist.org). For example, from 37 Felidae species, the fate of domestic cat causes no concern; but all the wild felids are either rare or endangered species. Moreover, the area inhabited by wild Felidae species is constantly reducing, thus many of them are on the Red List. The situation with other Carnivora families is not much better than with Felidae. The list of endangered Mustelidae species is also quite impressive (Schreiber et al. 1989).
As the methods traditionally used for in situ or ex situ preservation of mammalian species are expensive, cryobanking became popular as an approach for these species preservation (Amstislavsky et al., 2008).
Another reason for the popularity of reproductive biotechnology is the rapid increase of the overall number of transgenic and knockout mouse strains, which creates the need for their archiving. About 1 000 rat strains and 10 000 mouse strains are now available (Abbott, 2004).
The majority of these strains have been created by transgenesis, mutagenesis and knockout of individual genes. It is assumed that the total number of mouse strains will reach 300 000 over the next two decades. According to the metaphor of A. Abbott, geneticists are expecting for a “deluge” of mutant mouse strains in some close future. Foreign experts are unanimous that the problem of the drastic increase in the number of laboratory animal strains could be alleviated by cryobanking of these genetic resources. The Federation of International Mouse Resources (FIMRe) supplied with cryoarchive was established. Frozen semen and embryos are collected in these cryobanks, thus needed strain may be re-established by embryo transfer and (or) artifical insemination. The FIMRe unites the leading Animal Resource Centers of North America, Europe, Japan, and Australia.
This article briefly describes our experimental results of the joint project of the two Institutes of the Russian Academy of Sciences (Institute of Cytology and Genetics; Institute of Systematics and Ecology of Animals) and the University of Kuopio (Institute of Applied Biotechnology). This project may be considered as an example of the use of embryotechnological approach, e.g. cryobanking, for the preservation of endangered mustelid species.
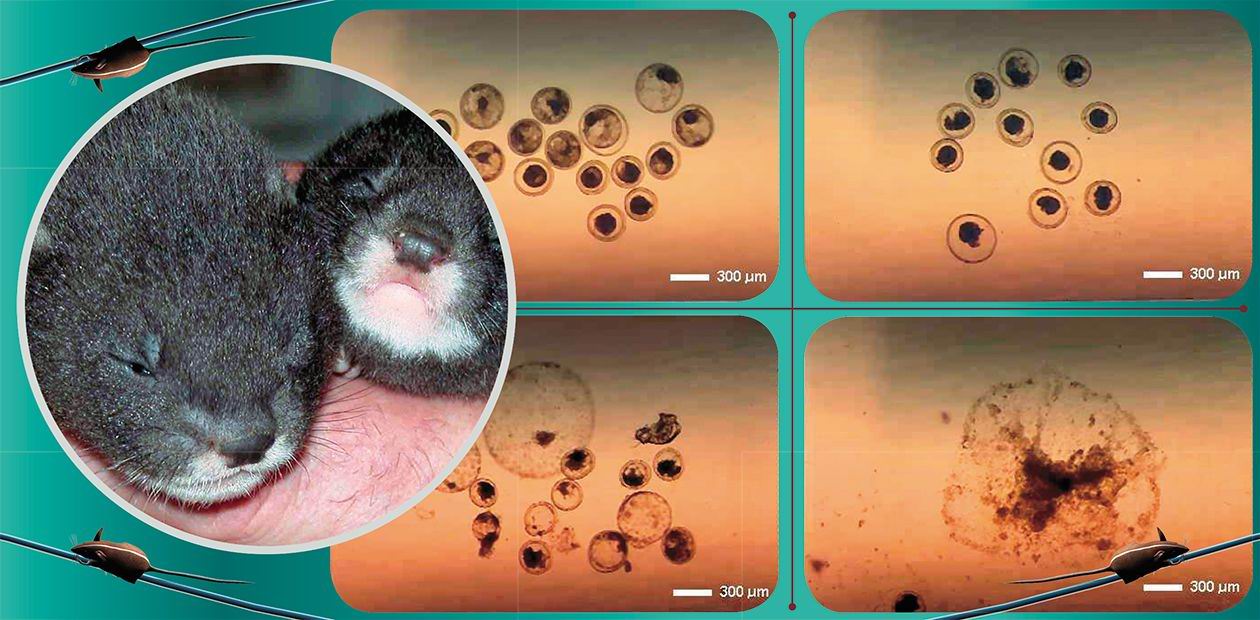
Initially this project involved polecats and, in part, stoats. The goal of the first stage was to demonstrate that the mustelid embryos could survive cryopreservation at the temperature of liquid nitrogen; it is noteworthy, that no positive result has been known in the world by the moment when this project started. Moreover, there had been no method applicable for freezing Carnivora embryos except for the only successful attempt by B. Dresser et al. (1988), who obtained live term kittens after transfer of frozen-thawed domestic cat embryos.
The goal of the first stage was to develop suitable methods of collection and transfer of mustelid embryos. The European polecat (Mustela putorius) and its domesticated form, the ferret (Mustela putorius furo) were chosen as the experimental models, as these are the closest relatives of the endangered European mink. In addition, we performed the first experiments on embryo cryopreservation with Muselidae embryos, i.e.: polecat/ferret and stoat (Mustela erminea) embryos (Amstislavsky et al., 1996; Kizilova et al., 1998).
We performed experiments on embryo transfer in mustelids on the unique Mustelidae farm founded by the Ternovsky couple in the late 1960s – early 1970s in Academgorodok (Ternovskaya et al., 2006). The experiments on embryo transfer between domestic ferrets and European polecats are rather illustrative. However, the first attempts to get live term kits after transfer of the frozen-thawed polecat embryos that had undergone complete procedure of freezing – cryopreservation – thawing were a failure.
At this point of the project we finally realized how sensitive Carnivora embryos are and understood why all the previous ever performed attempts to cryopreserve canid and felid embryos were a failure. One of the reasons of this is that the Carnivora embryos contain numerous lipid droplets, which are sensitive to cryopreservation procedures. Embryos of rodents and of the majority of farm animals contain fewer lipid droplets. This is the reason why the attempts made all over the world to obtain offspring after cryopreservation and subsequent embryo transfer of Carnivora embryos have almost always failed.
By that time we had gained considerable knowledge in Mustelidae reproductive biology and experience in the mammalian embryo transfer. Moreover, we have improved a biological model of embryo transfer in polecats. Thereat S. Ya. Amstislavsky was invited to the Institute of Applied Biotechnology (University of Kuopio, Finland) to participate in the project of embryo transfer and cryopreservation in polecats.
The author had earlier noticed that the most severe (visible in a light microscope) structural damages in the polecat and stoat embryos after freezing, cryopreservation and thawing appeared only at the stage of washing off the cryoprotectant. This inspired us to omit this stage at all and to transfer the embryos into the uterus straightly from cryoprotective medium, but to minimize the amount of this medium which inevitably will be transferred together with embryos. The problem associated with this approach was the toxicity of glycerol and DMSO (dimethyl sulfoxide), used as cryoprotectant. Good luck helped to overcome this problem. That time, ethylene glycol, a less toxic cryoprotectant “became fashionable” and we decided to try it instead of DMSO and glycerol. Moreover, in Novosibirsk we optimized the procedure of embryo transfer in such a way that it was possible to put embryos into the uterus in a minimal volume of the medium. This “know-how” became really important when we decided to transfer embryos directly into the uterus, skipping the stage of washing off the cryoprotectant.
At the very beginning of the Finnish stage of this international project, an encouraging preliminary result was obtained. We demonstrated that polecat embryos after freezing with ethylene glycol, cryopreservation, and thawing were able to develop in vitro up to the “implantation”, i.e. up to attachment to the bottom of the Petri dish (Amstislavsky et al., 2000).
Finally we got term kits after transfer of frozen-cryopreserved-thawed embryos. “Omitting washing off” cryoprotectant was more effective approach. Polecat term kits were also born after “washing off” cryoprotectant and subsequent transfer, but success rate was lower in this traditional method, if compared to the innovative first method (Lindeberg et al., 2003). In parallel, we thoroughly studied biological model of embryo transfer in this species and found out how many embryos should be transferred, which day of pseudopregnancy is the best to perform embryo transfer, how precisely the donor and recipient should be synchronized, e.t.c. All this was very important to make the project a success, as we have learned from the previous experiments with various mammalian species, including laboratory and farm animals, that the more embryos were available before freezing and the more optimized were embryo transfer protocols, the higher was the chance of the positive result (Amstislavsky et al., 1991).
After the press conference in 2001, our team became famous in Finland. The result that impressed journalists so much was really interesting. That time it was the world first success in cryopreservation of Carnivora embryos, beside mentioned above the only successful experiment with domestic cat. Photos of term polecat kits were published in many newspapers, including the well known Helsingin Sanomat.
Later we recommenced the embryotechnological experiments with Mustelidae embryos transfer on the Ternovsky’s farm. These works were supported in 2002 - 2006 by a joint project of Russian and Finnish Academies. Moving of our experimental activity back to Novosibirsk was caused by the very important fact: there are only four farms in the world, where endangered European mink is successfully bred in captivity. As early as 100 – 150 years ago, the European mink habitat covered the entire Europe and even spread up to the Ob river basin (Schreiber et al., 1989; Ternovsky, Ternovskaya, 1994). This huge range has nowadays reduced to three small areas, inhabited correspondingly by western population in Pyrenees, southern population in delta of Danube, and northeastern population in Russia and Belarus.
However, even though the scientific community and ecologists are aware of the problem, this species continues to decline.
The captive bred farm populations of European mink become the main guarantee that this species will not extinct. These individuals capable of reproducing in captivity makes it possible to study reproductive biology of this species and to conduct experiments on the re-introduction of this species back to wild (Ternovsky, Ternovskaya, 1994).
European mink is nowadays bred in captivity in Estonia, Germany and Spain; however, the pioneer in the field was Novosibirsk, Academgorodok. Even though investments into foreign farms (especially, into the recently constructed modern center in Spain) are incomparably larger than investments into Ternovsky’s farm, the mink birth rate remains the highest in the latter ( Ternovskaya et al., 2006). Tiit Maran, the head of European mink farm in Estonia, and one of the most eminent specialists in the area of European mink conservation (http://www.lutreola.ee/), keeps being really impressed by the fact of superiority of Ternovsky’s farm despite the shortage of funding.
Thus, it was the Ternovsky’s farm where we, since the year 2002, have performed our research on the reproductive biology of the European mink and have explored possibilities of embryotechnological approaches for this species conservation. We tried to develop an effective model of embryo transfer of European mink embryos into the hybrids between the European minks and polecats. Such hybrids, named khonoriks and nokhoriks, have been produced for many years on the Ternovsky’s farm. Khonorik, the hybrid between a male polecat and a female European mink was for the first time obtained by the Ternovskys (Ternovsky, Ternovskaya, 1994). This method has become widely accepted and the word khonorik is nowadays well known. A reverse hybrid between a male European mink a female polecat was for the first time obtained in 2001; so far, nokhorik has been obtained only at the Ternovsky’s farm.
Hybridization between polecats and European mink sometimes occurs in wild and considered as one possible reason of the European mink rapid decline. We tried to turn the negative phenomenon of polecat hybridization to the advantage of declining species, and this approach was a success.
In 2002—2004 we transferred a total of 56 European mink embryos into 9 khonorik females and got 28 kits as a result. In addition, 16 embryos of the European mink were transferred into 3 nokhorik females, 8 term kits were born. Thus, the success rate was 50% (Amstislavsky et al. 2004; 2006).
In one experiment, we transferred five European mink and five polecat embryos into the same khonorik recipient female and got four term mink kits and one polecat kit in the same litter. This result impressed the editors of the scientific journal Reproduction Fertility and Development so much, that they placed the photo of this litter on the cover page of the issue, where this and other our results were published (Amstislavsky et al., 2006).
The situation is indeed unique: “the womb”, or, more precisely, uterine brothers and sisters belong to different species—a polecat and a mink.
Despite this success, there are still problems to be solved. For example, the rate of success after transfer of frozen-thawed mustelid embryos is still low: only 11 % frozen-thawed polecat embryos develop to term after being kept in cryobank. This is considerably lower success rate if compared to success rate of frozen—thawed embryo transfer in most farm and laboratory animal species.
In addition, a direct embryo transfer between European mink and polecat has not yet resulted in term kits. The attempts of interspecies embryo transfer even between closely related species encounter problems associated with differences in hormonal and immune mechanisms of pregnancy regulation in different animal species. To overcome the interspecies barrier, these species-specific mechanisms should be further elucidated.
Note in conclusion that this project has allowed us to pioneer the feasibility of mustelid embryos cryopreservation and also allowed us to develop a model of embryo transfer of endangered mustelid species, the European mink. It should be also noticed, that some of our achievements have been recently appreciated. In 2003, this approach has been approved at the First International Conference on the Conservation of the European Mink (Logrono, Spain) and got an official recognition in the Conference resolutions. In 2008 we were awarded a grant from the Russian Foundation for Basic Research for studying the interspecies reproductive barrier between the European mink and polecat and for investigating the possibilities to overcome this barrier. First results have been obtained (Amstislavsky et al., 2008). This success gives hope for the further advance in this direction and provides some positive background for the final development of an integrated package of reproductive technologies which will include embryo cryobanking as one of key elements. This should be accompanied by further study of species-specific reproductive biology in the European mink.
Another reason for our optimism is the fact that the American relative of European mink, black-footed ferret (Mustela nigripes), which was once considered as endangered and even extinct, is nowadays out of danger (Grenier et al., 2007). This recent success was promoted by the years of thorough investigation of the reproductive biology of this mustelid species, by the numerous attempts of its reintroduction into wild and by the improvement of methods of its breeding in captivity. Reproductive technologies were considered as the core elements of this integrative approach.
References
Abbott A. Geneticists prepare for deluge of mutant mice // Nature.—2004.—V. 432.—P. 541.
Amstislavsky S. et al. Conservation of the European mink (Mustela lutreola): Focus on reproduction and reproductive technologies // Reprod. Domest. Anim.—2008.—V. 43.—Рp. 502—513.
Amstislavsky S. et al. Embryo cryobanking for conserving laboratory and wild animal species // Scand. J. Lab. Anim. Science.—1996.—V. 23.—Рp. 269—277.
Amstislavsky S. et al. Embryo development and embryo transfer in the European mink (Mustela lutreola), an endangered mustelid species // Reprod. Fertil. Dev.—2006.—V. 18. — Рp. 459—467.
Amstislavsky S. et al. Ex-situ preservation of Mustelidae: primer of application of genetic resource bank concept with the use of polecats as the model species // Scientifur.—2000.—V. 24.—Рp. 45—58.
Amstislavsky S. et al. Transfer of European mink (Mustela lutreola) embryos into hybrid recipients // Theriogenol.—2004.— V. 62.—Рp. 458—467.
Amstislavsky S.Ya. Embryotechnology Approaches to Preservation of Declining Mammalian Species: Doctoral Dissertation in Biology.—Novosibirsk, 2006.
Amstislavsky S.Ya. et al. Methods of Biotechnology in the Practice of Animal Breeding.—Novosibirsk: ICG, 1991 [in Russian].
Dresser B. L. et al. First successful transfer of cryopreserved feline (Felis catus) embryos resulting in live offspring // J. Exp. Zool.—1988.—V. 246.—Рp. 180—186.
Grenier M. B. et al. Rapid population growth of a critically endangered carnivore // Science.—2007.—V. 317.—P. 779.
Kizilova E.A. et al. The effect of cryopreservation on the morphology of polecat blastocysts // Ontogenez.—1998.—V. 29.—Pp. 1—8.
Lindeberg H. et al. Surgical recovery and successful surgical transfer of conventionally frozen—thawed embryos in the farmed European polecat (Mustela putorius) //Theriogenology.— 2003.—V. 60.—Рp. 1515—1526.
Lindeberg H. et al. Surgical transfer of in vivo produced farmed European polecat (Mustela putorius) embryos // Theriogenology. — 2002. — V. 57. — Рp. 2167—2177.
Schreiber A. et al. Weasels, Civets, Mongooses and Their Relatives. An Action Plan for Conservation of Mustelids and Viverrids.—USA: Kelvin Press, 1989.
Ternovskaya Y. et al. Strategies for European mink preservation: Proceedings of the International Conference on the Conservation of the European Mink, 2003, Logrono, Spain // Gobierno de la Rioja, 2006.—Рp. 267—279.
Ternovsky D.V., Ternovskaya Yu.G. The Ecology of Weasels.— Novosibirsk: Nauka, 1994.