Truth about Phage Therapy or a Memo to Physicians and Patients
The first clinical experiments with bacteriophages began a century ago. It seemed that the new method was bound to be a success: the approach looked faultless from a scientific standpoint and the results of its application were most promising. Then why did the interest in the therapeutic use of bacteriophages disappear in the subsequent decades? Why did this interest emerge once again, and why has this idea not been implemented to the fullest so far? Both medical practitioners and their patients should understand well not only the essence of this promising therapy, but also its merits and flaws
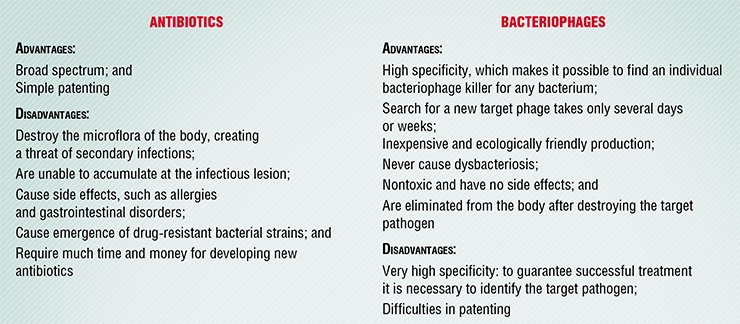
Bacteriophages are not just common drugs. They are neither simple chemicals, like antibiotics and most other medicines, nor true living organisms since they, like all the other viruses, are able to reproduce only in the cell of their host. Actually, bacteriophages are nano-automatons with their own genetic program, which can penetrate into a bacterial cell and reproduce there causing its destruction.
Therefore, standard pharmacological approaches to bacteriophages are not always satisfactory. Although phage preparations are now produced and applied in medicine, our knowledge about the diversity of these viruses, about mechanisms of their interaction with bacteria, and about competition with their cognates is still insufficient to use their full therapeutic potential.
Safe and Efficient
Phage therapy emerged almost immediately after bacteriophages were discovered; however, expanded trials of these antibacterial tools were launched in the Soviet Union only in the late 1930s. The trials proved the efficiency of bacteriophages as preventive measures against dysentery and cholera epidemics, and using them for healing wounds and curing pyoinflammatory diseases demonstrated their potential as an alternative to antibiotics.
However, the results achieved at that time were often contradictory: in some cases the phages immediately inhibited the progression of infection but sometimes they were of no use. The specialists grasped the reason right away: the treatment was successful only when they used phages that were able to infect the particular bacterial strain that had caused the target infection. Thus, it was necessary to isolate the pathogen that caused the epidemic, assay the available phages for their ability to inhibit this agent, and produce the most efficient bacteriophage as a drug.
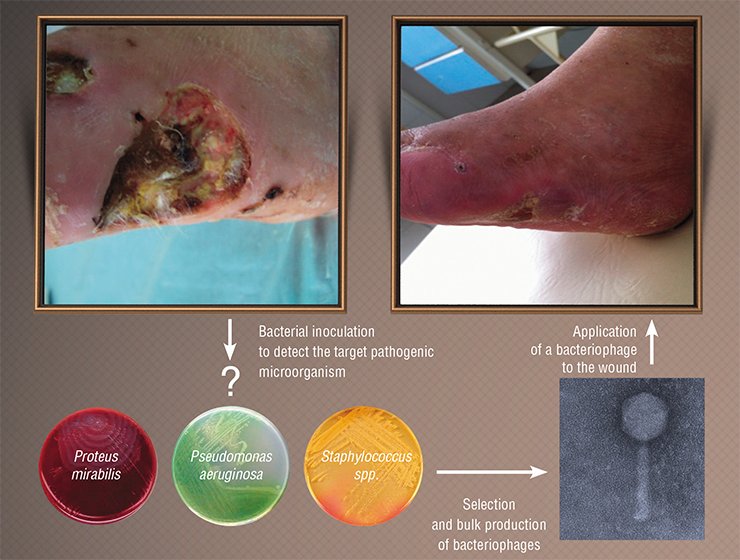
Unfortunately, the results of the studies performed in the Soviet Union were not properly documented and described in scientific literature; moreover, they were conducted not in compliance with the currently recognized protocols for clinical trials. Nonetheless, the major results of that work were undoubted: phages demonstrated their safety and high efficiency in real situations. Since then they have been used in clinical practice in this country along with common therapeutic tools.
Initially, the FDA restrained the spread of this approach, trying to apply the regularity rules approved for ordinary drugs. However, the protests of both therapists and patients came into play, and the method was approved with common precautions, i. e. selection of healthy donors and performance of the procedure by specialists and in healthcare facilities. The method has recently become widespread in the United States and shows good results. It is likely that only the prejudice of physicians still hinders the use of fecal microbiota transplantation in several European countries; in Russia, this treatment is available only at the Center for New Medical Technologies in Novosibirsk Akademgorodok
With the advent of antibiotics, western countries lost interest in phages; however, the emergence of antibiotic-resistant bacterial strains made several countries begin to elaborate phage preparations and conduct clinical trials, which in fact were the same as the ones that had been performed in the Soviet Union. The new results confirmed the safety of bacteriophage preparations, which was confirmed by the Food and Drug Administration (FDA).
In the United Kingdom, experiments on treating chronic otitis caused by have proved to be successful. Under the Phagoburn project, seven medical centers in France, Belgium, and Switzerland are involved in the clinical trials of a phage cocktail for preventing infections in burn injuries. Several United States companies (Intralytix, Enbiotix, and AmpliPhi) report testing their original phage cocktails for a wide range of diseases, though none of these large-scale clinical trials has been completed yet.
What is a “medicinal bacteriophage?”
In Russia, bacteriophage preparations are available in pharmacies, but unlike other drugs with their precise chemical formula and concentration of the components, a bacteriophage preparation is a nonstandard solution containing live virus particles. Preparations that have the same name but were manufactured at different facilities or at different times may differ in their composition and/or ratio of phages.
All the differences are determined by the specificity of the phage selection procedure and their production. Bacteriophages are selected according to their ability to lyse an individual bacterial isolate; then a mixture of phages is grown on a specified bacterial culture, and goes into production, i. e. bacteriophages are grown in voluminous reactors (fermenters) with the help of bacterial strains.
As a result, a drug that can kill the necessary bacterial strain is created. For example, the Pseudomonas aeruginosa bacteriophage contains the phages that kill P. aeruginosa, but the physician does not know either the number of phages in the preparation or what phages it contains, what P. aeruginosa strains it can kill, and whether it is appropriate for a particular patient. The preparation will have an excellent effect if the patient is infected with the same bacterial strain as was used for phage production; otherwise, the only hope is that since the phage cocktail contains many components, one of the bacteriophages may be specific to the target pathogen.
It is believed that the overwhelming majority of microorganisms in their natural environment (flow conditions) exist at the interface of two media as “biofilms,” a sort of “colonies” with a specific spatial and metabolic structure. The bacterial cells in such “microbial cities” are submerged into an extracellular mucous matrix formed of polymeric substances secreted by cells. The biofilm can be attached to the surface of either inanimate objects (for example, calculi, catheters, or joint implants) or to a part of an animate object (intestinal walls, teeth, or skin). This mode of existence provides many advantages for microorganisms; in particular, the biofilm microflora, owing to the mucous matrix, is much less affected by various adverse factors, such as ultraviolet radiation, dehydration, or antibiotics. The matrix also protects the bacteria against the attacks of bacteriophages and host immune cellsThus, it does not pay to buy a bacteriophage in a drugstore for self-treatment. It is up to the doctor to prescribe the treatment and drugs. The range of diseases susceptible to bacteriophage therapy is wide, including trophic ulcers, burn and wound infections, as well as various infections of respiratory, urogenital, gastrointestinal organs, and bones. In these cases, the causative agents are notorious bacteria, such as Staphylococcus aureus, Pseudomonas aeruginosa, pathogenic Escherichia coli strains, salmonellas, Proteus, and streptococci, including their drug-resistant variants. In fact, it is possible to find naturally occurring bacteriophages against any bacteria, including those that cause plague and anthrax. Bacteriophages can also be used to prevent communicable bacterial diseases; for example, they were successfully used in kindergartens to prevent a dysentery epidemic.
Bacteriophage preparations are administered either locally, to the lesion, or orally. Advertisements allege that phages can spread within the human body and pass from the stomach to bloodstream; however, there is no clear and unambiguous scientific proof yet. Note that a bacteriophage preparation may contain most different bacterial viruses with most diverse fates in the human body.
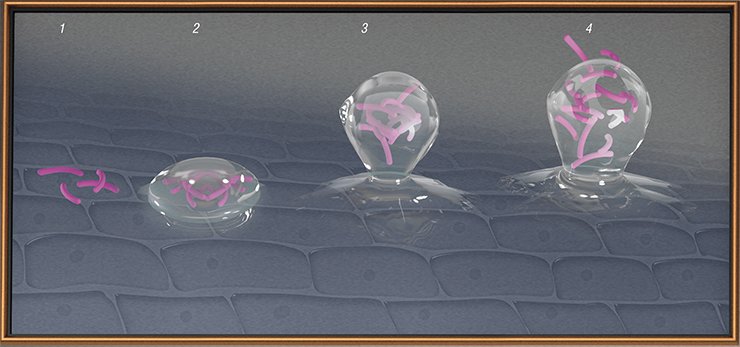
In certain situations, it is difficult for bacteriophages to hit their “victims.” For example, tuberculosis bacteria reside within the body cells where phages cannot get, while some bacteria form biofilms which are impenetrable for both phages and antibiotics. Then, to destroy the biofilms it is necessary to use enzymes synthesized by specially constructed phages.
In an infected organism, phages reproduce until the majority of sensitive bacterial cells are killed. The patient in whose body bacteriophages fight with bacteria will finally get well when his/her immune system starts to work in full force, and will further protect the body for a long time independently of whether the pathogen is present or not.
Besides, thanks to their specificity, bacteriophages do not kill “good” microorganisms, i.e. unlike antibiotics, they do not damage the human microbiome. It is now known that the intestinal flora disturbance may cause severe consequences, namely certain problems with the gastrointestinal tract, various allergies, and functional impairments of the central nervous system. Another advantage is that bacteriophages do not interfere with the effects of other therapeutics, and are not influenced by them either.
An individual cocktail for everyone
Why have bacteriophages failed to become the main tool for controlling infections, and there are frequent complaints of unsuccessful treatment in the social networks? This is explained in part by the administering of inadequate preparations. A decade ago, numerous “healthcare facilities” advertised stem cell preparations as drugs to cure any disease; now, extracts of “bacteria isolated from permafrost” and “preparations involving bacteriophages” (with no bacteriophages detectable) are intensively advertised. When buying a preparation, you should be sure that it was produced by a reliable manufacturer.
The main cause of treatment failures is an awkward selection of phages for individual patients. Each particular phage is efficient against one or a few bacterial strains, while an infection similar in its appearance, for example, angina, can be caused in individual patients by different streptococcal strains. To cure a patient, it is necessary to isolate the culture of the pathogen and test it for sensitivity to different phages, i.e. bacteriophage therapy should follow the pattern of personalized medicine. Alas, current medicine is not ready to do this yet.

The large collection of bacteriophages at the Institute of Chemical Biology and Fundamental Medicine contains unique strains that can fight the newly emerged but already widespread agents of hospital infections, such as the gram-negative bacteria Acinetobacter baumanii and Stenotrophomonas maltophilia. The phages that can lyse a broader range of bacterial strains, including drug-resistant variants, more efficiently than the bacteriophages available as commercial preparations have already been isolated and characterized. This set includes the bacteriophages of the Pseudomonas aureginosa, Proteus, Staphylococcus aureus, S. epidermidis, Klebsiella, and pathogenic Enterococcus. Genome sequencing of the most promising bacteriophages has allowed the researchers to find strains that differ from the known ones.
Clinicians participated in accumulating the expertise to use bacteriophages for treating infections that accompany the diabetic foot syndrome, osteomyelitis, and surgical wound infections. A few cases of curing respiratory infections caused by drug-resistant pathogens and urogenital diseases have been reported. For example, a 6-month-old girl with a congenital laryngeal abnormality was completely cured of severe tracheobronchitis, which had developed after tracheotomy, by inhalations of the Pseudomonas aureginosa bacteriophage. The pathogen that had caused her disease was identified as the Pseudomonas aureginosa strain, resistant to almost all antibiotics approved for infants. The girl received the bacteriophage twice a day for a week; after the pathogen and infection disappeared, the tracheotomy tube was removed; now the girl is quite well.
As for treating chronic drug-resistant urinary infections, it has been found that the bacteriophage should be administered directly onto the bladder. This cured a patient with post-surgery chronic cystitis caused by a “bouquet” of antibiotic-resistant enterobacteria. A set of specific bacteriophages had been administered for 10 days on a daily basis; the urine tests after the treatment showed no pathogenic flora
The experience of the Soviet Union, Georgia, and Poland has shown that a successful use of bacteriophages requires not only a clinical facility, but also a laboratory production site with a collection of phages and the staff skilled in identifying bacteria as well as in selecting and isolating bacteriophages for individual patients.
The question here is whether large-scale production of bacteriophage preparations is reasonable. The answer is yes, because the problem of narrow specialization of bacteriophages is solved in part by making phage cocktails containing several (sometimes, several tens of) different phages that can hit different target pathogen strains. Evidently, it is easier and faster to select the necessary phage cocktail for an individual rather than to test many individual phages from a large collection.
For all that, bacteriophages are not likely to replace antibiotics completely: these preparations are complementary and applicable to different situations. When a patient is seriously ill, with a good reason to suspect a bacterial infection, there is no time for experiments in selecting the proper phage preparation. The only satisfactory solution then is a broad-spectrum antibiotic.
The Russian Federation has currently the largest-scale production of bacteriophages. Mikrogen, a research and production facility, and the world leader in this area, manufactures a wide range of phage preparations. Therapeutic bacteriophages are also produced in Georgia, at the Eliava International Phagotherapy Center, which comprises both production facilities and a clinic with a vast collection of bacteriophages. European and the United States clinics, where phage therapy has not been officially approved yet, cooperate with this center under a medical tourism program. The Phage Therapy Unit with the Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences produces bacteriophage preparations for experimental clinical application when treating patients who are non-sensitive to antibiotics
However, bacteriophage therapy is preferable when you deal with a chronic infection or with a disease caused by multidrug resistant bacteria. In the case of chronic illnesses, such as otitis, the physician has enough time to administer a phage cocktail or to select a specific phage. Another example is a post-surgery infection with an antibiotic-resistant bacterial strain, which causes rapid deterioration of the patient’s state; here phage therapy can be the only option.
Wide experience in the clinical use of bacteriophages acquired over the last 100 years demonstrates the promising future of phage medical technologies. Further efforts of the experts working in this area, in combination with synthetic biology tools, will certainly create preparations with incomparably higher efficiency than that of the currently available phage cocktails.
However, several factors unrelated to science hinder the advances in designing and producing “medicinal” bacteriophages. The fact is that bacterial viruses are very easily reproduced, which offers exciting possibilities for their counterfeiting, thereby infringing on the rights of bona fide manufacturers. The requirements for phages as therapeutics have not been established yet either. It is only clear that they should be different from the requirements for synthetic drugs. Bacteriophage genomes are diverse; so, if a personalized approach is used, they should be selected individually.
Still, biotechnologists as well as researchers and physicians hope that these safe and efficient preparations will take their rightful place in the toolkit of therapies for infectious diseases.
References
Aleshkin A. V. Bacteriophages in infectious pathology: past, present, and future, in: Lectures on the Study and Administration of Bacteriophages, Ulyanovsk, 2016, pp. 11–51.
Bacteriophages: Biology and Administration (E. Kutter and A. Sulakvelidze, eds.), Moscow: Nauchnyi Mir, 2012.
Górski A. et al. Phages targeting infected tissues: novel approach to phage therapy // Future Microbiol. 2015. V. 10. P. 199—204.
Kozlova Yu. N., Repin V. E., Anishchenko V. V., et al. The Pseudomonas aeruginosa bacteriophage strain used as a major component for manufacturing an aseptic against this bacterium, patent no. 2455355, 2012.
Kozlova Yu. N., Morozova V. V., Tikunova N. V., et al. The Staphylococcus aureus SA20 bacteriophage strain used to destroy the biofilms formed by the bacteria of the genus Staphylococcus, Russian patent no. 2565824, 2015.
Międzybrodzki R. et al. Clinical aspects of phage therapy // Adv. Virus. Res. 2012. V. 83. P. 73—121.
Morozova V. V., Kozlova Yu. N., Tikunova N. V., et al. The Citrobacter freundii CF17 bacteriophage strain that is able to lyse Citrobacter freundii pathogenic strains, Russian patent no. 2565559, 2015.
Tikunova N. V. and Morozova V. V. Phage display based on filamentous bacteriophages: application to the selection of recombinant antibodies // Acta Naturae. 2009. N. 3. P. 6–15.
Tikunova N. V. and Vlassov V. V. Bacteriophages – the enemies of our enemies. Science First Hand. 2013. N. 3. P. 30–41.
Reproductions from the monograph Healing Wounds with Bacteriophages (Moscow: Narkomzdrav USSR, Medgiz, 1941) are used















